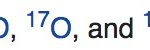Dioxygen It occurs in three isotopic forms such as in molecular state, it exists as O2 and is called dioxygen or simply oxygen. All the dioxygen in the atmosphere is believed to be due to the photosynthesis taking place in green plants in the presence of sunlight. H2O + x CO2 → (CH2O)x + xO2 Sunlight … [Read more...] about Dioxygen
The p-Block Elements
Chemical Properties of Group 16 Elements
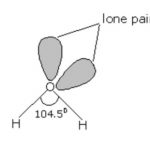
Chemical Properties of Group 16 Oxygen shows anomalous behaviour. This is due to its small size, high electronegativity, high ionisation enthalpy and absence of d-orbitals in its valence shell. Due to the absence of d-orbitals in oxygen, its covalency is limited to four. The valence shell can be expanded using vacant d-orbitals and hence covalence exceeds four. Both … [Read more...] about Chemical Properties of Group 16 Elements
Physical Properties of Group 16 Elements
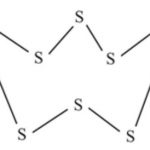
Chalcogens The elements oxygen (O), sulphur (S), selenium (Se), tellurium (Te) and polonium (Po) constitute group 16 elements of the periodic table. These are named as oxygen family after the name of the first member of the group. The first four elements of this group are collectively known as chalcogens (meaning ore forming elements) because many metal ores occur as … [Read more...] about Physical Properties of Group 16 Elements
Oxoacids of Phosphorus
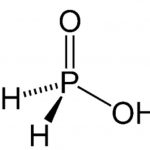
Oxoacids of Phosphorus – The p-Block Elements – Class 12 Some Important Oxoacids of Phosphorus (1) Phosphinic acid or Hypophosphorous acid, H3PO2 Preparation of Phosphinic acid or Hypophosphorous acid (1) It is prepared by heating phosphorus with alkalies like barium hydroxide when precipitate of barium phosphate is formed. The precipitate is separated and heated … [Read more...] about Oxoacids of Phosphorus
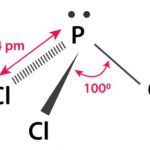
Halides and Oxides of Phosphorus
Halides and Oxides of Phosphorus – The p-Block Elements – Class 12 Halides of Phosphorus Phosphorus forms two types of halides i.e., phosphorus trihalides (covalency of P =3) PX3 (X = F, CI, Br, I) and phosphorus pentahalides PX5 (X = F, Cl, Br) (covalency of P=5). With chlorine it forms: (1) Phosphorus trichloride (2) Phosphorus … [Read more...] about Halides and Oxides of Phosphorus