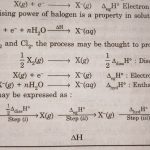Chemical Reactivity The halogens react readily with metals and non-metals to form halides. Fluorine is the most reactive of all the halogens. The reactivity of the halogens decreases down the group. The high reactivity of halogens is due to the following reasons : (i) Low dissociation enthalpies All the halogens have very low dissociation enthalpies. As a result, … [Read more...] about Chemical Properties of Group 17 Elements
The p-Block Elements
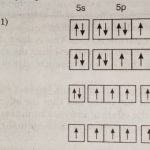
Physical Properties of Group 17 elements
Group 17 Elements Group 17 of the periodic table contains five elements : fluorine (F), chlorine (Cl), bromine (Br), iodine (I) and astatine (At). These are named as halogens. The name halogens is derived from two Greek words halo meaning sea salt and gens meaning born i.e., sea salt produce because the first three members occur as salts (chlorides, bromides and iodides) in … [Read more...] about Physical Properties of Group 17 elements
Sulphuric Acid
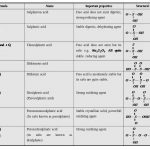
Oxoacids of Sulphur Sulphuric Acid Manufacture of Sulphuric Acid Sulphuric acid can be manufactured by Contact process. The process involves the following steps: (i) Preparation of sulphur dioxide Sulphur dioxide is prepared by burning sulphur or iron pyrites in excess of air. S+O2 → SO2 4FeS2 + 11O2 → 2Fe2O3 + 8SO2 Iron pyrites (ii) Oxidation of … [Read more...] about Sulphuric Acid
Allotropes of Sulphur and Sulphur Dioxide
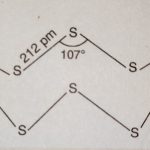
Sulphur occurs in the free state as well as in the combined state. Allotropes of Sulphur Sulphur exists in numerous allotropic forms of which three forms are the most important. These three main allotropic forms are: (i) Rhombic sulphur (iii) Plastic sulphur (ii) Monoclinic sulphur (i) Rhombic sulphur or α-Sulphur (i) It is the common … [Read more...] about Allotropes of Sulphur and Sulphur Dioxide
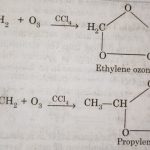
Ozone
Ozone Ozone is an allotropic form of oxygen. It is present in the upper atmosphere (about 20 km above the surface of the earth). It is believed to be formed in the upper atmosphere by the action of ultraviolet rays on oxygen as 3O2 + Ultraviolet rays → 2O3 ΔH298K = 142.7 kJ mol-1 Ultraviolet rays, which are harmful to human beings, are absorbed by oxygen to form … [Read more...] about Ozone