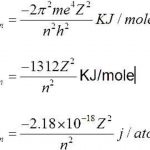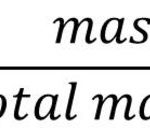Hydrogen Spectrum Atomic spectrum of hydrogen consists of a number of lines which have been grouped into 5 series :Lyman, Balmer, Paschen, Brackett and Pfund. Any given sample of hydrogen gas gas contains a large number of molecules. When such a sample is heated to a high temperature or an electric discharge is passed, the hydrogen molecules splits into hydrogen … [Read more...] about Hydrogen Spectrum
Class 11
Bohr’s Model of an Atom
Neils Bohr, a Danish physicist in 1913 proposed a new model of atom. This new model is called Bohr's Model of Atom. Postulates of Bohr's model of an Atom 1) An atom consists of a small, heavy positively charged nucleus in the centre and electrons revolve around it in circular orbit. 2) The electrons revolve only in those orbits which have a fixed value of energy. These … [Read more...] about Bohr’s Model of an Atom
Limiting Reagent
The reactant which reacts completely in the reaction is called limiting reactant or limiting reagent. The reactant which is not consumed completely in the reaction is called excess reactant . Question : 3 g of H2 react with 29 g of O2 to form H20.Which is the limiting reagent ? Answer: Thus O2 is present in excess.Hence H2 is the limiting reagent. … [Read more...] about Limiting Reagent
Balancing Of A Chemical Equation
Balancing of a chemical equation means making the number of atoms of each element equal on both sides of the equation. The methods of balancing equation are: 1) Hit and Trial Method: The simplest method to balance a chemical equation is by hit and trial method. Step 1 : Write down the correct formula of the reactants and products with plus sign in between with an … [Read more...] about Balancing Of A Chemical Equation
Empirical and Molecular Formula
Calculation of Percentage Composition The percentage of any element or constituent in a compound is the number of parts by mass of that element or constituent present in 100 parts by mass of the compound. Step 1 : Calculate the molecular mass of the compound from its formula by adding the atomic masses of the element present. Step 2 : Calculate the … [Read more...] about Empirical and Molecular Formula