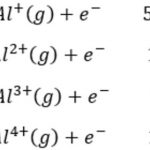The period of an element corresponds to the principal quantum number of the valence shell. The block of an element corresponds to the type of orbital which receive the last electron. The group of an element is predicted from the number of electrons in the valence shell or/and penultimate shell as follows: a)For s block elements ,group number is equal to the number of … [Read more...] about Prediction of period, group and block of a given element
Class 11
Ionization Enthalpy
If sufficient energy is supplied, electrons may be removed resulting in the formation of a positively charged ion. The minimum amount of energy required to remove the most loosely bound electron from an isolated gaseous atom so to convert it into gaseous cation is called ionisation enthalpy. It is represented by Δ i H This process may be represented as M (g) + Δ i H … [Read more...] about Ionization Enthalpy
Division of elements into s, p, d and f block
Elements in the long form of periodic table have been divided into four blocks i.e. s ,p ,d and f. This division is based upon the name of the orbitals which receives the last electron. S block elements 1) Elements in which the last electron enters the s orbital of their respective outermost shells are called s block elements. 2) s sub shell has only 1 orbital which can … [Read more...] about Division of elements into s, p, d and f block
Electronic configuration Of Elements
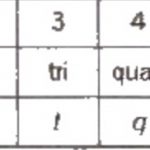
(1) The names are derived directly from the atomic numbers using numerical root for 0 and numbers from 1-9 and adding the suffix ium. The roots for the numbers 0-9 are: (2) In certain cases, the names are shortened.bi ium and tri ium are shortened to bium and trium and enn nil shortened to ennil. (3) The symbol of the element is then obtained from the first letters of … [Read more...] about Electronic configuration Of Elements
Modern Periodic Table
Modern periodic law states that physical and chemical properties of the elements are periodic function of the atomic numbers i.e. if the elements are arranged in order of their increasing atomic numbers, the elements with similar properties are repeated after certain regular intervals. Atomic mass depends upon the number of protons and neutrons in the nucleus whereas atomic … [Read more...] about Modern Periodic Table