When a balanced chemical equation not only indicates the quantities of the different reactants and products but also indicates the amount of heat evolved or absorbed, it is called thermochemical equation. Fractional coefficients may be used in writing a thermochemical equation. H2 ( g ) + ½ O2 ( g ) ---------> H2O ( l ) +285.8 KJ mol-1 H2 ( g ) + ½ O2 ( g ) … [Read more...] about Thermochemical Equation
Class 11
Exothermic And Endothermic Reaction
Exothermic reactions These are those reactions which are accompanied by the evolution of heat. The quantity of heat produced is shown along with the products with a plus sign. For Ex: C ( s) + O2 (g) ------> CO2 ( g) + 395.3 kJ H2 (g) + ½ O2 (g) --------> H2O ( l ) + 285.8 KJ N2 ( g ) + 3 H2 ( g ) --------> 2 NH3 ( g ) + 92.4 KJ CH4 ( g ) + 2 O2 … [Read more...] about Exothermic And Endothermic Reaction
Measurement Of Change In Internal Energy and Enthalpy
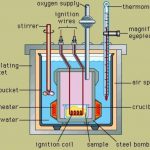
The experimental technique of measuring energy changes accompanying any chemical or physical process is called calorimetry. These measurements are generally carried out under 2 condition 1) at constant volume ( ΔU or qv ) 2) at constant pressure ( ΔH or qp) Measurements of ΔU Internal energy change is measured experimentally using an apparatus called Bomb … [Read more...] about Measurement Of Change In Internal Energy and Enthalpy
Heat Capacity
Heat capacity of a system is defined as the amount of heat required to raise the temperature of the system through 1°C . If q is the amount of heat supplied to a system and as a result ,if the temperature of the system rises from T1 and T2 ,then the heat capacity of the system is given by C = q / ( T2 -T1 ) C= q / Δ T Since the heat capacity varies with temperature … [Read more...] about Heat Capacity
Enthalpy
According to first law of thermodynamics, q= ΔU + PΔV If the process is carried out at constant volume , ΔV=0 qv = ΔU where v indicates constant volume. Internal energy change is the heat absorbed or evolved at constant volume. ΔU is a state function, therefore qv ,is also a state function. If the process is carried out at constant pressure, the work of … [Read more...] about Enthalpy