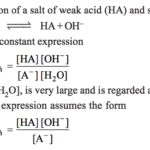
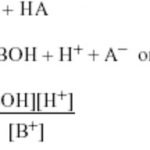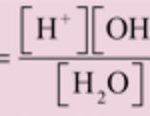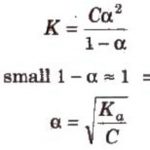Salt of weak Acid and strong base a) Hydrolysis Constant b) Degree of Hydrolysis A‾ + H2O OH‾ + HA c 0 0 original molar conc c(1-h ) ch ch Molar conc at equilibrium c) pH Salt of strong Acid and weak base a) Hydrolysis Constant BA + H2O BOH + HA B+ + A‾ + … [Read more...] about Calculation of Hydrolysis Constant, Degree of Hydrolysis and pH of Salt Solution
Chemistry
Salt Hydrolysis
Salt hydrolysis is defined as the process in which a salt reacts with water to give back the acid and the base. Salt +water ----------> Acid + Base BA + H2O ---------> HA + BOH All salts are strong electrolytes and thus ionize completely in the aqueous solution. (1) If the acid produced is strong and the base produced is weak. B+ + A‾ + H2O -------> H+ + … [Read more...] about Salt Hydrolysis
pH
A solution may be neutral, acidic or alkaline. Kw = 1 × 10-14 at 298 K To express the acidity or alkalinity of a solution, it is sufficient to express only the H3O+ ion concentration. Sorensen , in 1909 , suggested a convenient method of expressing the H3O+ ion concentration in terms of pH. The symbol has been taken from the Danish word potenz de hydrogen ion … [Read more...] about pH
Dissociation Constant and Ionic Product of Water
Pure water is poor conductor of electricity. Water is a weak electrolyte i.e. it is ionized to a very small extent as: H2O H+ + OH‾ H2O + H2O H3O+ + OH‾ This ionization is called self ionization of water. where Keq is the dissociation constant of water. Kw is the ionic product of water. Ionic product of water may be defined as the product of the … [Read more...] about Dissociation Constant and Ionic Product of Water
Strengths of Acids And Bases
According to Arrhenius concept, an acid is defined as a substance which when dissolved in water gives H+ and a base is defined as a substance which when dissolved in water give OH‾ ions. Greater the number of H+ ions produced in the aqueous solution, the stronger is the acid. Greater the number of OH‾ ions produced in the aqueous solution, the stronger is the base. As … [Read more...] about Strengths of Acids And Bases