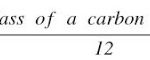Question 1 What is a chemical formula? Question 2 Give few examples of formulae of elements? Question 3 Give few examples of formulae of compounds? Question 4 Define molecular mass? Chemical Formula A chemical formula represents the composition of a molecule of the substance in terms of the symbols of elements present in the molecule. Formulae of Elements For … [Read more...] about Molecular mass
Class 9
Molecules
Question 1 What is a molecule. Give example? Question 2 How do atoms usually exist? Question 3 Why atoms form molecules or ions? Question 4 Define covalent bond? Question 5 What is meant by molecule of an element? Question 6 What is meant by molecule of a compound? Question 7 Define the term atomicity? Question 8 What are monoatomic molecules.Give … [Read more...] about Molecules
Atomic Mass
Question 1 How is the size of an atom indicated? Question 2 Define atomic mass unit? Question 3 What is the mass of hydrogen atom? Question 4 Name the element used as a standard for atomic mass scale? Atomic Mass of an Element Actual masses of the atoms of the elements are very very small. For Example : The atom of hydrogen has a mass of 1.6727 x 10-27 kg. It is … [Read more...] about Atomic Mass
Atoms and Elements
Question 1 Define atoms? Question 2 What is atomic radius? Question 3 What do you mean by symbol of an element? Question 4 Name the unit in which the radius of an atom is usually expressed? ATOMS All the matter is made up of atoms. An atom is the smallest particle of an element that can take part in a chemical reaction. Atoms of most of the elements are … [Read more...] about Atoms and Elements
Dalton’s Atomic Theory
Question 1 Name the scientist who gave atomic theory of matter? Question 2 State the various postulates of Dalton's Atomic theory of matter? Question 3 Give drawbacks of Dalton's Atomic theory of matter? Dalton's Atomic Theory Dalton put forward his atomic theory of matter in 1808. Postulates (1) All the matter is made up of very small particles called atoms. (2) … [Read more...] about Dalton’s Atomic Theory