Question 1 What are acids? Give example? Question 2 What are organic acids. Give example? Question 3 What are mineral acids. Give example? Question 4 What are strong acids? Question 5 What are weak acids? Question 6 What are dilute acids? Question 7 What are concentrated acids? Question 8 Which gas is usually liberated when an acid react with a metal. How … [Read more...] about Acids and their Properties
Class 10
Effects of Oxidation Reaction in Everyday Life
Question 1 Explain the term corrosion with an example? Question 2 Explain the term rancidity? Question 3 What are anti-oxidants. Give example? Question 4 Why anti-oxidants are added to fat and oil containing foods? Question 5 Why food containing oil and fat are packed in nitrogen? Question 6 State methods to prevent the food from getting rancid? Effects of … [Read more...] about Effects of Oxidation Reaction in Everyday Life
Oxidation and Reduction Reaction
Question 1 What are oxidation reactions? Question 2 What are reduction reactions? Question 3 What are redox reactions? Question 4 What are oxidising agent? Question 5 What are reducing agent? Question 6 Give example of redox reaction? Oxidation and Reduction Reaction Oxidation Addition of oxygen to a substance or removal of hydrogen from a … [Read more...] about Oxidation and Reduction Reaction
Double Displacement Reaction
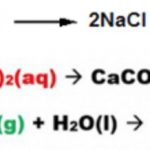
Question 1 What are double displacement reaction? Question 2 Give example of double displacement reaction? Question 3 What are precipitation reactions? Double Displacement Reaction Those reactions in which two compounds react by an exchange of ions to form two new compounds. are called Double Displacement Reactions. For Example : AgNO3(aq) + NaCl(aq) → AgCl(s) + … [Read more...] about Double Displacement Reaction
Displacement Reaction
Question 1 What are displacement reaction? Question 2 Give the reactivity series of various elements? Question 3 Give few example of displacement reaction? Displacement Reaction Those reactions in which one element takes the position of other element in a compound. or More reactive metal displaces a less reactive metal. Reactivity Series K Na Ca Mg Al Zn NI Pb H … [Read more...] about Displacement Reaction