Hydrogen is the most abundant element in the universe.
The giant planets such as Jupiter and Saturn contain mostly hydrogen.
Hydrogen constitutes about half of the mass of Sun and stars. The extremely high temperature of the Sun brings about fusion of hydrogen atoms liberating large amount of energy
It is the third most abundant element on the surface of the globe.
Earth does not possess enough gravitational pull to retain the light hydrogen molecule, therefore it is not found in our atmosphere.
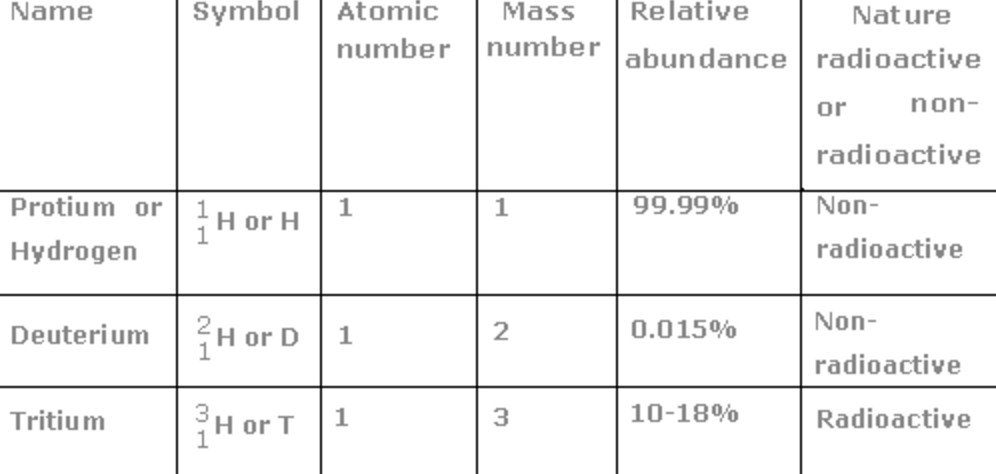
Isotopes of Hydrogen
Isotopes are the different atoms of same element which have the same atomic number but different mass number.
Hydrogen has three isotopes. These are protium, deuterium and tritium.
The most abundant isotope of hydrogen is protium.
0.0156 % is deuterium. It is mostly in the form of HD.
Tritium being unstable because of its radioactive nature occurs only in traces.
All the three isotopes of hydrogen have same atomic number therefore all have only one electron in their only shells and one proton in the nucleus.
Since the mass number of the three isotopes are different, therefore, they differ from one another in number of neutrons.
1) Protium : Atomic number = 1, Mass number =1. Its nucleus has only one proton and one electron revolving around the nucleus in its only Shell i.e. K shell.
2) Deuterium or heavy hydrogen : Its natural abundance is 0.0156%. it is usually prepared by the electrolysis of heavy water. Atomic number = 1, mass number = 2 .
Its nucleus has 1 protons and 1 neutrons while one electron is present in the K shell.
3) Tritium : It is the least abundant of all the isotopes of Hydrogen. It is formed in the upper atmosphere by reaction induced by cosmic rays. It is radioactive with a short half-life. it decays by β emission with no γ radiation.
It is prepared artificially by the bombardment of nitrogen or an isotope of lithium with neutrons.
Its mass number is 3. Its nucleus has 1 protons and 2 neutrons while one electron is present in the L shell.
Isotope Effect
The three isotopes of hydrogen have the same atomic number and electronic configuration, they have similar chemical properties. But owing to their different masses, the rate of equilibrium constant of these reactions are different.
Difference in properties due to difference in atomic masses is called isotope effect.
Due to different masses, the physical properties of these isotopes are quite different.
Uses of Tritium
Tritium is used to make thermonuclear devices and for carrying out researches in fusion reaction as a means of producing energy.
Tritium gas is usually stored by UT3, which on heating to 673 K releases T2.
2UT3 ———–> 2U + 3 T2
It is widely used as a radioactive tracer since it is relatively cheap and easy to work with.
Leave a Reply