Question 1 Name few applications of chemical effect of electric current?
Question 2 What is electroplating?
Question 3 Name the metal which is electroplated on car parts such as bumpers and bicycle handle bars?
Question 4 Which metal is electroplated on iron making cans used for storing food?
Question 5 What is the purpose of electroplating?
Question 6 Why does a brand new bicycle have shining handlebar and wheel rims?What will happen if these are accidentally scratched?
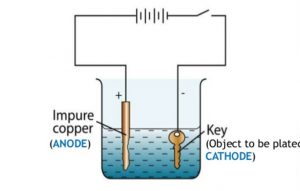
Question 7 With the help of a labelled diagram ,describe how an iron key can be electroplated with copper?
Question 8 Which property of chromium metal make it suitable for electroplating it on car bumper, bath taps and bicycle handle bars?
Question 9 What is chromium plating?
Question 10 What is tin plating?
Question 11 What is nickel plating?
Question 12 What is copper plating?
The chemical effects of electric current is used in industries or factories for the following purposes:
1) Electroplating metals.
2)Purification of metals
3)Production of certain metals from the ore
4)Production of chemical compounds
5)Decomposing chemical compounds
In electroplating the word electro stands for electric current and plating means the act of covering a metal object with a thin layer of coating of a different metal
Electroplating
The process of depositing a thin layer of a desired metal over a metal object with the help of electric current is called electroplating.
Electroplating is done for
1)Protection from corrosion
2)Testing for decorative purpose
For example :Bathroom taps made of iron or steel are electroplated with chromium metal to prevent their corrosion or rusting. It gives a shining appearance to the bathroom taps due to which they look more attractive. The metal objects are usually electroplated with chromium, nickel, silver ,gold or copper metals.
When a metal object is electroplated with chromium, it is called chromium plating
When a metal object is electroplated with tin, it is called tin plating
When a metal object is electroplated with Nickel it is called Nickel plating
When metal object is electroplated with silver it is called silver plating
When a metal object is electroplated with gold it is called gold plating
When a metal object is electroplated with copper it is called copper plating
1)The metal object on which electroplating is to be done is made the negative electrode: It is connected to the negative terminal of the battery.
2)The metal whose layer is to be deposited is made the positive electrode :It is connected to the positive terminal of the battery.
3)A water soluble salt of the metal to be deposited is taken as the electrolyte.
For electroplating an iron object with copper metal :
1)The iron object is made negative electrode that is cathode.This means that the iron object is connected to the negative terminal of the battery.
2)A copper plate is made positive electrode that is anode. This means that a copper plate is connected to the positive terminal of the battery.
3)Copper sulphate solution is taken as electrolyte.
Activity: Electroplating of an iron object with copper
Take 250 ml of distilled water in a clean beaker dissolved to tablespoons of copper sulphate in it. This will give us a blue coloured copper sulphate solution. Add a few drops of dilute sulphuric acid to copper sulphate solution to make it more conducting.Take a copper plate of about 10 cm * 4 cm size and a door key made of iron. Clean the surface of copper plate and iron key by rubbing with Sandpaper. Then wash them with water and dry them.
1)Immerse the cleaned copper plate in the copper sulphate solution in the beaker. Connect the copper plates to the positive terminal of a battery through a switch. This copper plate becomes the positive electrode or anode.
2)Immerse the clean iron key also in copper sulphate solution at a small distance from the copper plate. Connect the negative terminal of the battery to the iron key. This iron key becomes the negative electrode.
3)Switch on the electric current by closing the switch. Allows the current to pass for about 15 minutes.
4)Remove the copper plate and iron key from the copper sulphate solution and look at them carefully. We will find that the copper plate has dissolved a little and the iron key has got a reddish layer of copper metal all over its surface.Thus the iron key has been electroplated with copper.
The copper sulphate solution has copper metal in the dissolved form.The copper sulphate solution consists of free positively charged copper ions and negatively charged sulphate ions. when electric current is passed through copper sulphate solution ,then the following changes takes place :
1)The dissolved copper metal present in the copper sulphate solution as positively charged copper ions gets attracted to the negatively charged electrode iron key. The positively charged copper ions lose their positive charge on coming in contact with the negatively charged Ion key and from copper atoms.These copper atoms deposit on the iron key to form a thin layer of copper metal all over the surface of iron key.In this way, copper metal in the electrolyte comes out of the solution and forms a thin layer on the iron key.
2)The copper metal of positively charged copper plate electrode dissolves by forming positively charged copper ions.The copper ions thus formed go into the copper sulphate solution. In this way the loss of copper ions from copper sulphate solution is made up and the process continues. Since the copper ions are taken out from the solution at the negative electrode but put into solution at the positive electrode therefore the concentration of copper sulphate solution remains constant.
During copper plating of an iron key, copper metal is transferred from the copper plate to the iron key through the copper sulphate solution.During electroplating with copper, copper metal gets transferred from positive electrode to the negative electrode.
where is anser
Answer is in notes itself.
Very helpful I must say but umm…the iron key ie the cathode should be connected to the positive terminal of the battery while the anode (copper) should be connected to the negative terminal of the battery. God bless you as you effect the correction. Thank you
is it a displacement reaction or not?