Contents
- 1 Osmosis
- 2 Semipermeable Membrane
- 3 Demonstration of Osmosis
- 4 Osmotic Pressure
- 5 Osmotic pressure-a Colligative Property
- 6 Determination of Molar Mass from Osmotic Pressure
- 7 Conditions for Getting Accurate Value of Molar Mass
- 8 Biological Significance of Osmosis
- 9 Isotonic Solutions
- 10 Hypertonic and Hypotonic Solutions
- 11 Examples of Osmosis
- 12 Reverse osmosis an desalination of sea water
Osmosis
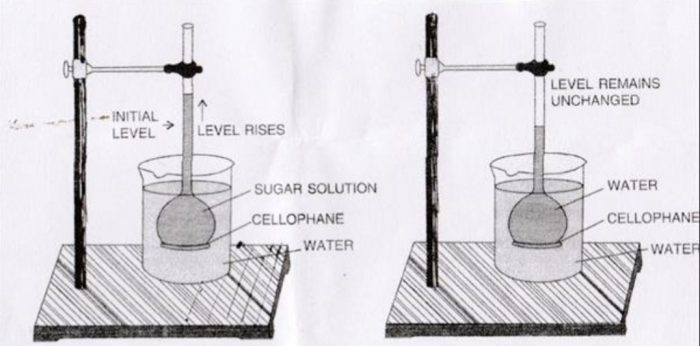
Consider an aqueous solution of sugar placed in an inverted thistle funnel having a semipermeable membrane (SPM) such as animal bladder or parchment paper, attached to its bottom. The thistle funnel is lowered into a beaker containing water. The membrane is such that it allows only the molecules of the solvent and not of the solute to pass through it.
Thus, there will be movement of water molecules from pure solvent into the solution. As a result, water passes into the thistle funnel and level of solution in the thistle funnel rises gradually. This process is called osmosis.
The phenomenon of the flow of solvent through a semipermeable membrane from pure solvent to the solution is called osmosis.
Osmosis can also take place between the solutions of different concentrations. In such cases, the solvent molecules move from the solution of low solute concentration to that of higher solute concentration.
| Osmosis | Diffusion |
| The process of osmosis takes place through a semi-permeable membrane. | No semi-permeable membrane is needed for the diffusion process. |
| The osmosis involves the movement of the solvent molecules only. | In diffusion both the solute and the solvent molecules can move. |
| In osmosis, molecules of solvent move from a region of lower concentration of solution into a region of higher concentration. | In diffusion, the molecules move from a region of higher concentration into the region of lower concentration.
|
| Osmosis is limited to solutions only. | Diffusion is common is gases as well as in liquids. |
| Osmosis can be stopped or reversed by applying additional pressure on the higher concentration side. | It cannot be stopped or reversed. |
Semipermeable Membrane
A semipermeable membrane is one which allows only the solvent and not the solute to pass through it.
The film of gelatinous precipitates of cupric ferrocyanide, Cu2[(Fe(CN)6)]. These membranes appear to be continuous sheets or films, yet they contain a network of submicroscopic holes or pores. Small solvent molecules like water can pass through these holes but bigger molecules like solute cannot pass through these holes. These types of membranes are called semipermeable membranes (SPM)
Demonstration of Osmosis
a) Take two eggs. Remove the outer hard shell of the eggs by dissolving in dilute HCl.
b) Place one egg in distilled water and the other egg in a saturated sodium chloride solution.
c) After few minutes, it will be observed that the egg placed in water swells whereas the other placed in salt solution shrinks.
Reason
The skin of the egg acts as a semipermeable membrane.
a) In the first case, pure water will enter into the egg material due to osmosis. Therefore, the egg swells.
b) The water comes out of egg material when placed in saturated solution of sodium chloride. Therefore, the egg shrinks.
This is because osmosis occurs from higher concentration of solvent to lower concentration of solvent. When placed in pure water, the concentration of water is higher outside the egg. But when placed in a saturated solution of sodium chloride concentration of water is higher inside the egg.
Osmotic Pressure
When an aqueous solution of sugar is placed in an inverted thistle funnel and separated from pure water with the help of a semi-permeable membrane, there will be osmosis of water molecules into the solution of sugar. As a result, the level of solution in the stem of the funnel will rise and additional hydrostatic pressure will be exerted on the solution. This hydrostatic pressure will tend to oppose the inflow of solvent into the funnel. The level of solution will continue rising till a particular height. At this stage, equilibrium is reached and the hydrostatic pressure of the liquid exactly balances the tendency of the liquid to pass inward through the semipermeable membrane. Thus, there will be no more osmosis taking place.
The equilibrium hydrostatic pressure on the solution due to osmosis of the pure solvent into it is a measure of osmotic pressure.
It consists of two vessels connected by a semi-permeable membrane. These two compartments are fitted with water-tight frictionless pistons. Let us take solution in one compartment and pure solvent in the other compartment. Due to osmosis, there will be flow of solvent into the solution compartment through the semipermeable membrane. As a result, the piston on the solution side will tend to move outwards. To stop this movement of piston outwards, we have to apply pressure on the solution side. This pressure just sufficient to stop osmosis will be equal to the osmotic pressure.
Osmotic pressure may be defined as the excess pressure which must be applied to a solution to prevent the passage of solvent into it through a semipermeable membrane.
Osmotic pressure is the pressure applied to the solution to prevent osmosis. It is generally denoted by π.
Osmotic pressure-a Colligative Property
Van’t Hoff (1887) concluded that a dilute or ideal solution behaves like an ideal gas and the different gas laws are applicable to the dilute solutions as well.
Van’t Hoff observed that for dilute solutions, the osmotic pressure (π) is given as:
π = cRT
where c is the molar concentration of the solution (molarity). T is the temperature and R is the gas constant.
For a solution, at a given temperature, both R and T are constant.
π ∝ c
Since osmotic pressure depends upon the molar concentration of solution is therefore, a colligative property.
Determination of Molar Mass from Osmotic Pressure
According to Van’t Hoff equation,
π = cRT
c= n/V
where n is the number of moles of solute dissolved in V litre of the solution.
π = n R T /V
The number of moles of solute n may be given as wB / MB . Here wB is the weight of the solute and MB is its molar mass.
Substituting the value of n in the above expression,
π = wB R T / V MB
MB = wB R T / V π
Thus, the molar mass of the solute, MB can be calculated.
Conditions for Getting Accurate Value of Molar Mass
(i) The solute must be non-volatile.
(ii) The solution must be dilute, i.e., concentration of the solution in the solution should not be more than 5%.
(iii) The solute should not undergo either dissociation or association in the solution.
Biological Significance of Osmosis
The absorption of water by plants from the soil through the roots and its movement to different parts of plants is due to the process of osmosis. Plants and animal bodies are composed of very large number of cells. The cells contain a fluid (called cell sap) and the walls of the cells are made up of living cytoplasmic membrane which acts as a semipermeable membrane. These membranes allow water to pass through but block the passage of the enzymes and proteins that have been synthesised in the cell. The cell saps have generally higher osmotic pressure and, therefore, when the cells come in contact with water, there is tendency of water to enter into the cell due to osmosis. Therefore, the osmosis process helps the plants to absorb soil water and push it up to the stem and other parts of the plants and trees.
Isotonic Solutions
Different solutions have different vapour pressures. Consequently their osmotic pressures must also be different. When solutions are separated by a semipermeable membrane, the solvent molecules flow from the solution of lower osmotic pressure towards solution of higher
osmotic pressure. This continues till both the solutions attain the same osmotic pressure. At this stage, there is no further osmosis.
Such solutions having same osmotic pressure are called isotonic solutions or isosmotic solutions.
If two solutions have same concentrations, they must have same osmotic pressure at the same temperature. Thus, solutions of equimolar concentration at the same temperature
osmotic pressure, i.e., are isotonic.
Hypertonic and Hypotonic Solutions
If a solution has more osmotic pressure than some other solution, it is called hypertonic.
A solution having less osmotic pressure than the other solution is called hypotonic.
Thus, a hypertonic solution will be more concentrated with respect to other solution and a hypotonic solution will be less concentrated with respect to other solution.
A 0.91% (mass/volume) solution of sodium chloride (known as saline water) is isotonic with fluids inside human red blood cells (RBC). In this solution, the corpuscles neither swell nor shrink.
The medicines are mixed with saline water before being injected into the veins. Therefore, normal saline water is quite safe to inject intravenously.
However, the solutions having concentration more or less than 0.91% (mass/volume) are
not safe as :
(i) A pure sodium chloride solution with salt concentration less than 0.91% (mass/volume) is said to be hypotonic solution. When red blood cells are placed in this solution, water flows into the cells and they swell or burst.
(ii) A pure sodium chloride solution with salt concentration more than 0.91% (mass/volume) is said to be hypertonic solution. When red blood cells are placed in this solution, water flows out of the cells and they shrink.
People taking a lot of salt or salty food experience water retension in tissue cells and intercellular spaces because of osmosis. The swelling or puffiness is called edema. When red blood cells are placed in this solution, water comes out of the cells and they shrink.
Examples of Osmosis
(1) Carrots which have become limp because of water loss into the atmosphere can be placed into the water which makes them firm again. Water will move into them through osmosis.
(2) A raw mango placed in concentrated salt solution loses water via osmosis and shrivel into pickle.
(3) Wilted flowers revive when placed in fresh water due to osmosis.
(4) The preservation of meat by salting and fruits by adding sugar protects against bacterial action. A bacterium on salted meat or candid fruit loses water due to osmosis, shrivels and ultimately dies.
Reverse osmosis an desalination of sea water
The process of osmosis can be reversed if a pressure larger than the osmotic pressure is applied on the solution side. As a result, the solvent starts moving from solution towards the pure solvent through the semipermeable membrane. This process of movement of solvent through a semipermeable membrane from the solution to the pure solvent by applying excess pressure on solution side is called reverse osmosis.
Its written in very easy language and it becomes easier to understand the concepts after reading from here. It is really good .