Question 1 Show covalent bonding in hydrogen molecule?
Question 2 Show covalent bonding in oxygen molecule?
Question 3 Show covalent bonding in nitrogen molecule?
Question 4 Show covalent bonding in chlorine molecule?
Question 5 Show covalent bonding in water molecule?
Examples of covalent molecules
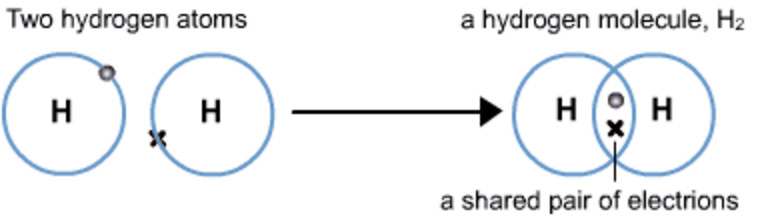
(1) Hydrogen molecule
The atomic number of hydrogen is 1. Hence hydrogen has one electron in its K shell and it requires one more electron to fill the K shell. So two hydrogen atoms share their electrons to form a molecule of hydrogen, H2 . This allows each hydrogen atom to attain the electronic configuration of the nearest noble gas, helium, which has two electrons in its K shell. We can depict this using dots or crosses to represent valence electrons.
The shared pair of electrons is said to constitute a single covalent bond between the two hydrogen atoms. A single covalent bond is also represented by a line between the two atoms.
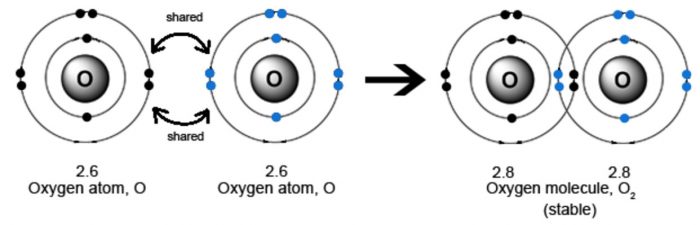
(2) Oxygen molecule
The atomic number of oxygen is 8.Its electronic configuration is 2 , 6. It has 6 electron in its outermost shell.It needs 2 more electron to complete its shell.Therefore O atom shares its electron with another oxygen atom.Thus there is a double covalent bond.
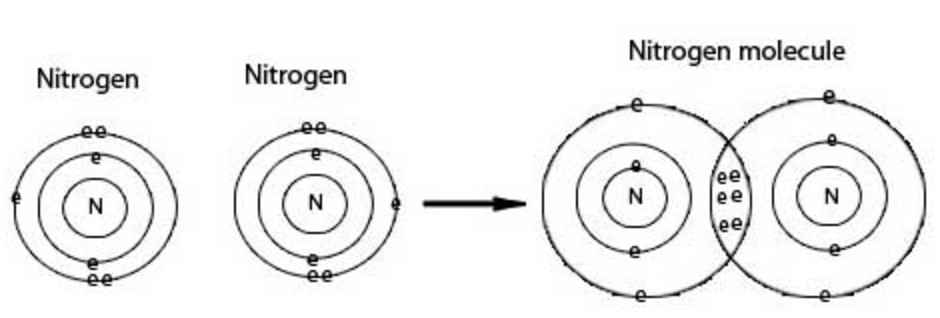
(3) Nitrogen molecule
The atomic number of nitrogen is 7.Its electronic configuration is 2 , 5. It has 5 electron in its outermost shell.It needs 3 more electron to complete its shell.Therefore N atom shares its electron with another nitrogen atom.Thus there is a triple covalent bond.
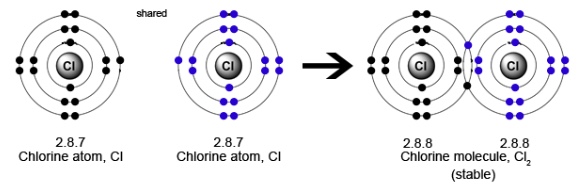
(4) Chlorine molecule
The atomic number of chlorine is 17 . Its electronic configuration is 2, 8 , 7 . It has 7 electron in its outermost shell.It needs 1 more electron to complete its shell.Chlorine gets this electron by sharing with another atom. So each chlorine atom shares one electron to form a chlorine molecule.Thus there is a single covalent bond.
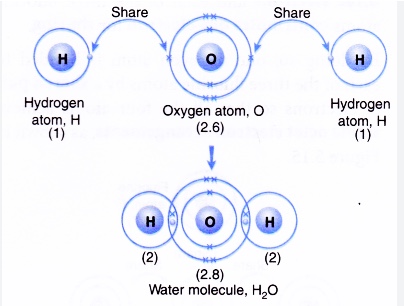
(5) Water molecule
The atomic number of Hydrogen is 1 .Its electronic configuration is 1.
The atomic number of oxygen is 8 .Its electronic configuration is 2 , 6.
Two hydrogen atoms each share their 1 electron with oxygen to form two covalent bonds and make a water molecule.
or
One oxygen atom shares its two electrons with two hydrogen atoms.
Thank you Shilpi. The website is clean and easy to follow.
Thanks mam for this answer but i need more examples of covelent molecules