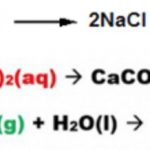
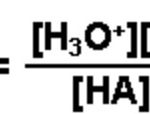Acids like HCl, H2SO4 ,HNO3 when dissolved in water dissociates almost completely thus producing a large number of H+ ions. These acids are called strong acids. Acids like CH3COOH , HF, H2CO3, H3PO4 dissociates only to a small extent in the aqueous solution giving small amount of H+ ions and hence are called weak acids. Bases like NaOH , KOH dissociate almost completely … [Read more...] about Dissociation or Ionization of Acids And Bases
weak acid
Common in Acids
Question 1 Why HCl show acidic behaviour whereas glucose and alcohol do not show acidic behaviour? Question 2 Why does an aqueous solution of an acid conduct electricity? Question 3 Why does dry HCl gas not change the colour of the dry litmus paper? Question 4 Why does distilled water no conduct electricity whereas rain does? Question 5 Compounds such as alcohol and … [Read more...] about Common in Acids
Acids and their Properties
Question 1 What are acids? Give example? Question 2 What are organic acids. Give example? Question 3 What are mineral acids. Give example? Question 4 What are strong acids? Question 5 What are weak acids? Question 6 What are dilute acids? Question 7 What are concentrated acids? Question 8 Which gas is usually liberated when an acid react with a metal. How … [Read more...] about Acids and their Properties