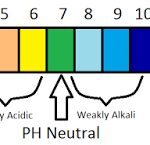
A solution may be neutral, acidic or alkaline. Kw = 1 × 10-14 at 298 K To express the acidity or alkalinity of a solution, it is sufficient to express only the H3O+ ion concentration. Sorensen , in 1909 , suggested a convenient method of expressing the H3O+ ion concentration in terms of pH. The symbol has been taken from the Danish word potenz de hydrogen ion … [Read more...] about pH
pH
Last Updated on By Mrs Shilpi Nagpal