Question 1 What does pH of a solution signify?
Question 2 You have 2 solutions A and B. The pH of solution A is 6 and pH of solution B is 8.Which of the solution has more hydrogen ion concentration?
Question 3 Three solutions A,B and C have pH values of 6,4 and 10.Which of the solution is highly acidic?
Question 4 The pH of a solution is 5.What will be its action on blue and red litmus?
Question 5 Name the scientist who discovered pH scale?
Question 6 Two solutions A and B have pH values of 3 and 9.Which will turn phenolphthalein from colourless to pink?
Question 7 What is the pH of neutral solution?
Question 8 Fresh milk has a pH of 6>how do you think the ph would change as it turns into curd?
Question 9 What effect does the concentration of hydrogen ion have on nature of the solution?
Strength of Acid and base: pH Scale
In pure water [H+] = [OH–]
1) Pure water + acid(produce H+) → Concentration of hydrogen ion(H+) increases Solution will have more of hydrogen ion(H+).
2) Pure Water + base (Produce OH-) → Concentration of OH- increases Solution will have more of OH- ions.
In 1909 Sorenson gave a scale known as pH scale. The strength of an acid as well as basic solution can be represented by making use of Hydrogen ion concentration (H+)in them. The pH of a solution is inversely proportional to hydrogen ion in it. High the concentration of hydrogen ion lower will be its pH. Lower the concentration of hydrogen ion Higher will be its pH.
P represents potenz means power whereas H represents concentration.
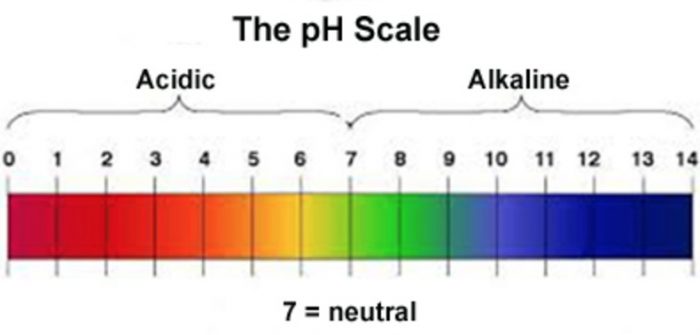
The strength of an acid or base is measured on a scale of numbers called pH scale (0-14).
1) Neutral Solution
pH=7
2) Acidic Solution
pH<7
More acidic a solution is lesser will be its pH.
Solution with pH=0,1,2,3 are strong acids.
Solution with pH=4,5,6 are weak acids.
Lower the pH stronger will be the acid.
3) Basic solution
pH>7
More basic a solution is more will be its pH.
Solution with pH=8,9,10 are weak base.
Solution with pH=11,12,13,14 are strong base.
Higher the pH, stronger will be a base.
Vinegar pH=4
Conc HCl pH=1
Conc NaOH pH=14
you are helping a lot of students madam
Mmm it was nice