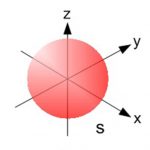
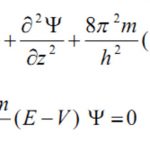An orbital is the region of space around the nucleus within which the probability of finding an electron of given energy is maximum. The probability at any point around the nucleus is calculated using schrodinger wave equation and is represented by the density of the points. Shape of s orbital For the coordinates( x, y, z) of the electron with respect to the nucleus, … [Read more...] about Shapes of Atomic Orbital
orbital
Quantum Numbers
Quantum Numbers An atom contains a large number of orbitals. These are distinguished from each other on the basis of their shape, size and orientation in space. These characteristics of an orbital are expressed in terms of three numbers, called principal, azimuthal and magnetic quantum number. Quantum numbers may be defined as a set of 4 numbers with the help of which we … [Read more...] about Quantum Numbers
Quantum Mechanical Model of an Atom
Quantum mechanics, as developed by Erwin Schrodinger in 1926, is based on the wave motion associated with the particles. For the wave motion of the electron in the three dimensional space around the nucleus, he put forward an equation known as Schrondinger wave equation. where ψ is the amplitude of the wave where the coordinates of the electrons are ( x,y,z) ,E is the … [Read more...] about Quantum Mechanical Model of an Atom