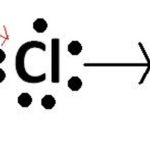Oxides and hydroxides All the alkali metals ,their oxides, peroxide and superoxide readily dissolve in water to produce corresponding hydroxides which are strong alkalies. 2 Na + 2 H2O -------------> 2 NaOH + H2 Na2O + H2O -------------> 2 NaOH Na2O2 + 2 H2O -------------> 2 NaOH + H2O2 2KO2 + 2 H2O -------------> 2 KOH + H2O2 + O2 Peroxides and … [Read more...] about Characteristics of Compounds of Alkali Metal
lattice enthalpy
Enthalpies of Reaction
Heat of reaction or enthalpy of reaction is a term used for the heat changes accompanying any reaction. Enthalpy of combustion The enthalpy of combustion of a substance is defined as the heat change when 1 mole of substance is completely burnt or oxidised in oxygen. CH4 ( g ) + 2O2 ( g ) ----------------> CO2 ( g ) + 2H2O ( g ) ΔcH= -890.4 KJ mol -1 Reaction … [Read more...] about Enthalpies of Reaction
Ionic Bond
When a bond is formed by complete transference of electrons from one atom to another so as to complete their outermost orbit by acquiring 8 electrons or 2 electrons in case of hydrogen, lithium etc and hence acquire the stable nearest noble gas configuration, the bond form is called ionic bond or electrovalent bond. On losing an electron, an atom becomes positively charged … [Read more...] about Ionic Bond