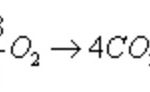Oxides and hydroxides All the alkali metals ,their oxides, peroxide and superoxide readily dissolve in water to produce corresponding hydroxides which are strong alkalies. 2 Na + 2 H2O -------------> 2 NaOH + H2 Na2O + H2O -------------> 2 NaOH Na2O2 + 2 H2O -------------> 2 NaOH + H2O2 2KO2 + 2 H2O -------------> 2 KOH + H2O2 + O2 Peroxides and … [Read more...] about Characteristics of Compounds of Alkali Metal
hydration enthalpy
Enthalpies of Reaction
Heat of reaction or enthalpy of reaction is a term used for the heat changes accompanying any reaction. Enthalpy of combustion The enthalpy of combustion of a substance is defined as the heat change when 1 mole of substance is completely burnt or oxidised in oxygen. CH4 ( g ) + 2O2 ( g ) ----------------> CO2 ( g ) + 2H2O ( g ) ΔcH= -890.4 KJ mol -1 Reaction … [Read more...] about Enthalpies of Reaction