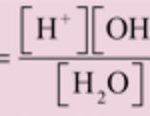Pure water is poor conductor of electricity. Water is a weak electrolyte i.e. it is ionized to a very small extent as: H2O H+ + OH‾ H2O + H2O H3O+ + OH‾ This ionization is called self ionization of water. where Keq is the dissociation constant of water. Kw is the ionic product of water. Ionic product of water may be defined as the product of the … [Read more...] about Dissociation Constant and Ionic Product of Water