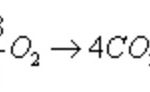Bond energy is the amount of energy released when 1 mole of bonds are formed from the isolated atoms in the gaseous state or the amount of energy required to dissociate one mole of bonds present between the atoms in the gaseous molecules. It is represented by Δ b H or Δbond H. For diatomic molecules like H2, O2, N2, Cl2, HCl, HF etc. the bond energies are equal to their … [Read more...] about Bond Enthalpy
Enthalpy of atomisation
Enthalpies of Reaction
Heat of reaction or enthalpy of reaction is a term used for the heat changes accompanying any reaction. Enthalpy of combustion The enthalpy of combustion of a substance is defined as the heat change when 1 mole of substance is completely burnt or oxidised in oxygen. CH4 ( g ) + 2O2 ( g ) ----------------> CO2 ( g ) + 2H2O ( g ) ΔcH= -890.4 KJ mol -1 Reaction … [Read more...] about Enthalpies of Reaction