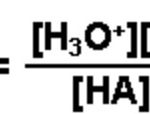Acids like HCl, H2SO4 ,HNO3 when dissolved in water dissociates almost completely thus producing a large number of H+ ions. These acids are called strong acids. Acids like CH3COOH , HF, H2CO3, H3PO4 dissociates only to a small extent in the aqueous solution giving small amount of H+ ions and hence are called weak acids. Bases like NaOH , KOH dissociate almost completely … [Read more...] about Dissociation or Ionization of Acids And Bases