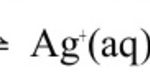If a sparingly soluble salt like AgCl is stirred with water ,only a small amount of it goes into solution while most of the salt remain undissolved. But little amount of salt dissolves, it gets completely dissociated into ions. when a sparingly soluble salt is added to water ,there exist a dynamic equilibrium between the undissolved solid salt and the ions which is … [Read more...] about Solubility Equilibrium and Solubility Product