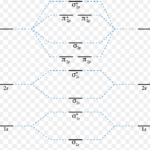
Energy level diagram for Molecular orbitals The first ten molecular orbitals may be arranged in order of energy as follow: σ(1s) <σ∗(1s) < σ(2s) <σ∗(2s) < π(2px) = π(2py) < σ(2pz) < π∗(2px) =π∗(2py) <π∗( 2pz) Relationship between electronic configuration and Molecular behaviour 1) Stability of molecules in terms of bonding and antibonding … [Read more...] about Energy level diagram for Molecular orbitals
bond order
Bond Characteristics
Bond Length When atoms come closer to each other, attraction takes place between them and ,therefore, the potential energy of the system keeps on decreasing till at a particular distance, the potential energy is minimum. If the atoms are further brought closer ,the repulsion start and therefore, the potential energy of the system begins to increase. At equilibrium … [Read more...] about Bond Characteristics