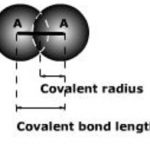
Bond Length When atoms come closer to each other, attraction takes place between them and ,therefore, the potential energy of the system keeps on decreasing till at a particular distance, the potential energy is minimum. If the atoms are further brought closer ,the repulsion start and therefore, the potential energy of the system begins to increase. At equilibrium … [Read more...] about Bond Characteristics
bond length
Valence Bond Theory
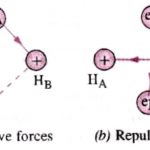
This theory was put forward by Heitler and London in 1927 and was further developed by Pauling and others. In terms of energy When the two atoms are far apart from each other there is no interaction between them. When they come closer to each other ,the new forces come into operation. These forces are of two types: 1)The forces of repulsion between the nuclei of … [Read more...] about Valence Bond Theory
Atomic Radius
Properties of the individual atoms Properties like valency ,atomic and ionic radii, ionisation enthalpy ,electron gain enthalpy, electronegativity are the properties of the individual atoms and are directly related to the electronic configuration. Properties of group of atoms Properties like melting point, boiling point ,heat of fusion and vaporisation, energy of … [Read more...] about Atomic Radius