Bond energy is the amount of energy released when 1 mole of bonds are formed from the isolated atoms in the gaseous state or the amount of energy required to dissociate one mole of bonds present between the atoms in the gaseous molecules. It is represented by Δ b H or Δbond H. For diatomic molecules like H2, O2, N2, Cl2, HCl, HF etc. the bond energies are equal to their … [Read more...] about Bond Enthalpy
bond energy
Valence Bond Theory
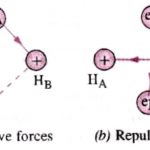
This theory was put forward by Heitler and London in 1927 and was further developed by Pauling and others. In terms of energy When the two atoms are far apart from each other there is no interaction between them. When they come closer to each other ,the new forces come into operation. These forces are of two types: 1)The forces of repulsion between the nuclei of … [Read more...] about Valence Bond Theory