Elements in the long form of periodic table have been divided into four blocks i.e. s ,p ,d and f. This division is based upon the name of the orbitals which receives the last electron. S block elements 1) Elements in which the last electron enters the s orbital of their respective outermost shells are called s block elements. 2) s sub shell has only 1 orbital which can … [Read more...] about Division of elements into s, p, d and f block
actinides
Electronic configuration Of Elements
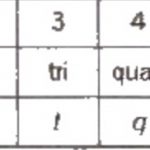
(1) The names are derived directly from the atomic numbers using numerical root for 0 and numbers from 1-9 and adding the suffix ium. The roots for the numbers 0-9 are: (2) In certain cases, the names are shortened.bi ium and tri ium are shortened to bium and trium and enn nil shortened to ennil. (3) The symbol of the element is then obtained from the first letters of … [Read more...] about Electronic configuration Of Elements
Mendeleev’s Periodic Law And Table
He examined the relationship between atomic weights of the elements and their physical and chemical properties. Among chemical properties, Mendeleev mainly concentrated on the compounds formed by the elements with hydrogen and oxygen because they are highly reactive and hence formed compounds with almost all the elements. The formulae of the hydrides and oxides formed by the … [Read more...] about Mendeleev’s Periodic Law And Table