Question 1 What is meant by rusting of iron? State two conditions necessary for rusting of iron? How rusting damages iron objects?
Question 2 What happens when an iron nail is kept immersed in copper sulphate solution?
Question 3 What happens when baking soda is added to vinegar? Give its chemical reaction?
Question 4 What happens when carbon dioxide is passed through lime water? Give the reactions involved?
Question 5 What happens when magnesium ribbon is burned in air? Name the type of changes which take place?
Question 6 What happens when magnesium oxide is dissolved in water? Write a word equation for this process?
Question 7 Why iron objects are painted frequently?
Question 8 Why iron pipes carrying water are coated with zinc?
Question 9 Why are tools and machine parts made of iron smeared with grease or oil?
Question 10 Why rusting of iron objects is faster in coastal areas than in deserts?
Question 11 What is meant by galvanisation? Why is it done?
Question 12 What is a chemical change? Give few examples from everyday life?
Question 13 What is meant by crystallisation? How crystals of copper sulphate are prepared by this method?
Question 14 State few characteristics of chemical changes?
Question 15 What is the importance of chemical changes?
Contents
- 1 Chemical changes
- 2 Characteristics of a chemical change :
- 3 Examples of chemical changes :
- 4 Burning of Magnesium Ribbon
- 5 Reaction Between Baking Soda and Vinegar
- 6 Reaction between Copper Sulphate Solution and Iron
- 7 Importance of Chemical Changes
- 8 Rusting of Iron
- 9 Conditions Necessary for Rusting
- 10 Prevention of rusting of iron
- 10.1 (1) Rusting of iron can be prevented by painting
- 10.2 (2) Rusting of iron can be prevented by applying grease or oil
- 10.3 (3) Rusting of iron can be prevented by galvanisation
- 10.4 (4) Iron is coated with chromium to prevent rusting
- 10.5 (5) Rusting of iron can be prevented by alloying it to make stainless steel
- 11 Crystallisation
- 12 Process of crystallisation
Chemical changes
Those changes in which new substances are formed, are called chemical changes. The properties of new substances formed in chemical changes are entirely different from those of the original substances.
During chemical change, a substance undergoes a change in its chemical composition (or change in chemical properties). Chemical changes are also called chemical reactions.
Characteristics of a chemical change :
(1) One or more new substances are formed in a chemical change.
(2) A chemical change is a permanent change. A chemical change usually cannot be reversed.
(3) A lot of energy (in the form of heat, light, etc.) is either absorbed or given out in a chemical change.
(4) Sound may be produced in a chemical change.
(5) A change in smell may take place or a new smell may be given off in a chemical change.
(6) A permanent change in colour may take place in a chemical change.
(7) A gas may be formed in a chemical change.
If we burn a piece of paper with a lighted match stick, then entirely new substances such as carbon dioxide, water vapour, smoke and ash are produced. So, the burning of paper is a chemical change. Heat and light are also given out during the burning of paper. The burning of paper is a permanent change which cannot be reversed. We cannot combine the products of burning of paper to form the original paper again. Burning is always accompanied by the production of heat and light.
Examples of chemical changes :
Souring of milk
Formation of curd from milk
Cooking of food (like rice and chapatis)
Spoilage of food
Change in colour of cut apple (cut brinjal or cut potato) on keeping in air
Photosynthesis
Digestion of food
Neutralisation reaction
Explosion of a firework (or cracker)
Burning of magnesium ribbon
Burning of fuels (like burning of wood, coal, kerosene, LPG and biogas)
Burning of dry leaves
Burning of candle wax
Burning of incense stick
Rusting of iron
Ripening of fruits
Reaction between vinegar and baking soda
Passing carbon dioxide gas through lime water (which produces calcium carbonate precipitate)
Reaction between copper sulphate solution and iron
Formation of manure (or compost) from leaves
Formation of biogas from animal wastes (like cow-dung).
When food gets spoiled, it produces a foul smell. This shows that new substances have been formed in the spoiled food which have foul smell. The spoilage of food is a chemical change.
The cut surface of an apple slice acquires a brown colour due to the formation of new substances by the action of oxygen (of air). So, the change in colour of a cut apple slice on keeping in air is due to a chemical change.
During photosynthesis, the plants combine carbon dioxide and water in the presence of chlorophyll and sunlight to form two new substances: glucose (food) and oxygen gas. Photosynthesis is a chemical change. In the process of digestion, the various food materials break down to form new substances which can be absorbed by the body. The process of digestion is a chemical change.
When an acid reacts with a base, then a neutralisation reaction takes place in which two new substances, salt and water, are formed, So, neutralisation is a chemical change. The explosion of a firework (like a cracker) is a chemical change because many new substances are formed in this process.
In a biogas plant, anaerobic bacteria digest (break down) the animal wastes (like cow-dung) and produce biogas whose major component is methane gas. The formation of biogas from animal wastes is a chemical change.
The burning of biogas is also a chemical change. This is because burning of biogas produces new substances like carbon dioxide and water vapour along with the evolution of heat.
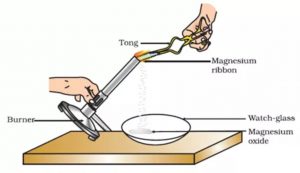
Burning of Magnesium Ribbon
A long and thin strip of magnesium metal is called magnesium ribbon. When a magnesium ribbon is heated, it burns in air with a brilliant white light to form a powdery ash called magnesium oxide. This magnesium oxide is an entirely new substance. So, the burning of magnesium ribbon is a chemical change.
Activity
Take a small piece of magnesium ribbon and clean it by rubbing its surface with a sand paper. Hold the magnesium ribbon at one end with a pair of tongs and bring its other end over the flame of a burner. The magnesium ribbon starts burning with a dazzling white light. Hold the burning magnesium ribbon over a watch glass so that the powdery ash being formed by the burning of magnesium collects in the watch glass. When magnesium ribbon burns in air, then the magnesium metal combines with the oxygen (of air) to form a new substance called magnesium oxide.
Magnesium + Oxygen——–> Magnesium oxide
Mg + O2 ——> MgO
Activity
Take magnesium oxide (ash) in a hard glass test-tube and add a small amount of water to it. Stir the magnesium oxide and water mixture carefully with a glass rod to obtain an aqueous solution of magnesium oxide.
(1) Take a strip of blue litmus paper and put a drop of magnesium oxide solution on it. The blue colour of litmus paper does not turn to red showing that magnesium oxide solution is not acidic.
(2)Now take a strip of red litmus paper and put a drop of magnesium oxide solution on it. The red litmus paper turns blue showing that magnesium oxide solution is basic in nature.
When we dissolve magnesium oxide in water, then magnesium oxide combines with water to form a new substance called magnesium hydroxide.
Magnesium oxide + water ——> Magnesium hydroxide
MgO + H2O ——> Mg(OH)2
The new substance ‘magnesium hydroxide’ formed during this change is a base which turns red litmus paper to blue. The dissolving of magnesium oxide in water is a chemical change.
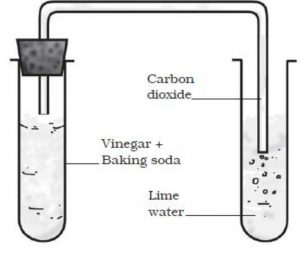
Reaction Between Baking Soda and Vinegar
When baking soda and vinegar are mixed together, then bubbles of carbon dioxide gas are formed . The reaction between baking soda and vinegar is a chemical change because it forms carbon dioxide as one of the new substances.
Take about 10 mL vinegar in a test-tube and add a pinch of baking soda to it. We will hear a hissing sound and see the bubbles of carbon dioxide gas coming out and rising in the test-tube.
Baking soda is sodium hydrogen carbonate and vinegar contains acetic acid. So, when baking soda and vinegar are mixed together, then a chemical change takes place between sodium hydrogen carbonate and acetic acid to form three new substances : sodium acetate, carbon dioxide and water.
Sodium hydrogen carbonate + Acetic acid ———-> Sodium acetate + Carbon dioxide + Water
Take some freshly prepared lime water in another test-tube. Pass carbon dioxide gas through lime water by using a glass delivery tube. We will see that lime water turns milky. Lime water is calcium hydroxide solution. When carbon dioxide gas is passed through lime water, then calcium hydroxide combines with carbon dioxide to form a white solid substance calcium carbonate.
Ca(OH)2 + CO2 ——> CaCO3 + H2O
The reaction between lime water and carbon dioxide gas is a chemical change because a new
substance calcium carbonate is formed during this change.
The turning of lime water milky is used as a standard test for carbon dioxide gas.
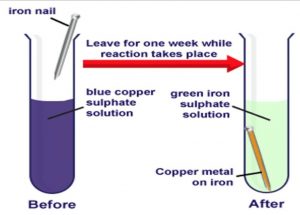
Reaction between Copper Sulphate Solution and Iron
When an iron object (like an iron nail, etc.) is kept immersed in the blue coloured solution of sulphate, then a chemical change takes place to form green coloured iron sulphate solution and a brown deposit of copper on the iron object (like nail).
Copper Sulphate + Iron ———> Iron Sulphate + Copper
CuSO4 + Fe ——-> FeSO4 + Cu
The reaction between copper sulphate (CuSO,)solution and iron (Fe) is a chemical change because it produces two new substances: iron sulphate (FeSO4) solution and copper (Cu). The common name of copper sulphate is blue vitriol.
Activity
Dissolve a little of copper sulphate in half test-tube of water. Add a few drops of dilute sulphuric acid to obtain a clear solution. This will give us a blue-coloured copper sulphate solution. Take a big iron nail and place it carefully in the test-tube containing copper sulphate solution. Keep the test-tube containing copper sulphate solution and iron nail aside for a few hours. The blue colour of copper sulphate solution fades gradually and ultimately changes to light green colour and a brown deposit is formed on the iron nail. We can take out the iron nail from the test-tube to see the brown deposit on it clearly.
Importance of Chemical Changes
Importance of chemical changes are given below:
(1) Metals are extracted from their naturally occurring compounds called ‘ores’ by a series of chemical changes.
(2) Medicines are prepared by carrying out a chain of chemical changes.
(3) The materials such as plastics, soaps, detergents, perfumes, acids, bases, salts, etc, are all made by carrying out various types of chemical changes.
(4) Every new material is discovered by studying different types of chemical changes.
The same substance can undergo a physical change or a chemical change depending, upon the conditions.
(1) The tearing of a sheet of paper into pieces of paper is a physical change but the burning of a sheet of paper is a chemical change.
(2)The melting of wax is a physical change but the burning of wax is a chemical change So, when a candle burns, then both physical and chemical changes take place. This is because when a candle burns, then some of the wax melts (physical change) and some of the wax burns (chemical change)
A Protective Shield of Ozone
There is a layer of ozone gas high up in the atmosphere.
The ozone layer protects us from the harmful ultraviolet radiations which come from the sun.
Ozone absorbs ultraviolet radiations coming from the sun and breaks down to form oxygen. The breaking down of ozone into oxygen is a chemical change.
If ultraviolet radiations were not absorbed by ozone layer, they would reach the earth’s
surface and cause harm to us and other living things. Ultraviolet radiations can cause skin cancer, damage our eyes and plants.
Rusting of Iron
When an iron object is left in damp air (or water) for a considerable time, it gets covered with a red-brown flaky substance called rust. This is called rusting of iron. During the rusting of iron, iron metal combines with the oxygen (of air) in the presence of water (moisture) to form a compound iron oxide. This iron oxide is rust.
Iron + Oxygen +Water ——> Iron Oxide
Fe + O2 + H2O ——> Fe2O3
Rust is iron oxide (Fe2O3). Thus, rust and iron are not the same substance. Rusting of iron is a chemical change.
Conditions Necessary for Rusting
Two conditions are necessary for the rusting of iron to take place:
(i) presence of oxygen (of air), and
(ii) presence of water or water vapour (called moisture).
Iron rusts when placed in damp air (or moist air), or when placed in water. Now, damp air (or moist air), also contains water vapour. Thus, damp air alone provides both the things, oxygen and water, required for the rusting of iron to occur. Ordinary water also supplies both the things, oxygen and water, needed for the rusting of iron.
(1) If the air at a place has a high moisture content (more water vapour) i.e. if the air at a place is more humid, then the rusting of iron becomes faster. The rusting of iron is faster in coastal areas (sea-side areas) because the air at those places contains more water vapour (or more moisture).
(2) The presence of salt in water makes the process of rusting of iron faster. Thus, an iron object will rust much faster when kept in sea-water (which is salty water) than when kept in fresh water (having no salts dissolved in it).
Prevention of rusting of iron
(1) Rusting of iron can be prevented by painting
When a coat of paint is applied to the surface of an iron object, then air and moisture cannot come in contact with the iron object and hence no rusting takes place. The window grills, railings, steel furniture, iron bridges, railway coaches, and bodies of cars, buses and trucks, are all painted regularly to protect them from rusting.
(2) Rusting of iron can be prevented by applying grease or oil
When some grease or oil is applied to the surface of an iron object, then air and moisture cannot come in contact with it and hence rusting is prevented.
The tools and machine parts made of iron and steel are smeared with grease or oil to prevent their rusting.
(3) Rusting of iron can be prevented by galvanisation
The process of depositing a thin layer (or coating) of zinc metal on iron objects is called galvanisation.
Galvanisation is done by dipping an iron object in molten zinc metal. A thin layer of zinc metal formed on the surface of an iron object protects it from rusting (because zinc metal remains unaffected by air and moisture). The iron sheets used for making buckets, drums, dust-bins and sheds (roofs) are galvanised to prevent their rusting. The iron pipes used in our homes to carry water are also galvanised to prevent rusting.
(4) Iron is coated with chromium to prevent rusting
This is called chrome-plating. Chromium metal is resistant to the action of air and moisture. So, when a layer of chromium is deposited on an iron object, then the iron object is protected from rusting.
Chromium-plating is done on steel furniture, taps, bicycle handle bars and car bumpers, etc, made of iron and steel to prevent them from rusting.
(5) Rusting of iron can be prevented by alloying it to make stainless steel
When iron is mixed (or alloyed) with carbon chromium and nickel, then stainless steel is obtained. Stainless steel is an alloy of iron.
Cooking utensils, knives, scissors and surgical instruments are made of stainless steel and do not rust at all.
Crystallisation
The process of evaporation (to dryness) is not a good technique of separation because:
(1) The soluble impurities do not get removed in the process of evaporation of a salt solution. So, the salt obtained by evaporation is not pure.
(2) The crystals of salts obtained by the process of evaporation are small. And the shape of crystals cannot be seen clearly.
Large crystals of pure substances can be obtained from their solutions by the process of Crystallisation.
Crystallisation is an example of a physical change.
The solid particles having flat surfaces, straight edges and regular shapes are called crystals.
The process of cooling a hot, concentrated solution of a substance to obtain crystals is called crystallisation. The process of crystallisation is used to obtain large crystals of a pure solid substance from the impure solid substance.
An impure solid substance usually contains two types of impurities: insoluble impurities and soluble impurities. The insoluble impurities are removed by filtering its solution whereas soluble impurities get removed during crystallisation.
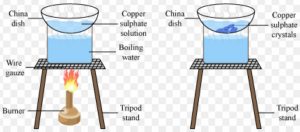
Process of crystallisation
Impure copper sulphate powder can be purified by the process of crystallisation to obtain large crystals of pure copper sulphate.
Take about 100 ml of water in a beaker and add a few drops of dilute sulphuric acid to it. Heat the water over a burner till it boils. Add copper sulphate powder slowly to the hot water with constant stirring. Continue to add copper sulphate till no more copper sulphate can be dissolved. This will give us a saturated solution of copper sulphate. Filter the hot saturated solution of copper sulphate to remove insoluble impurities. Allow the hot and concentrated
solution of copper sulphate to cool slowly. Do not disturb the solution when it is cooling. After some time, we will see large copper sulphate crystals at the bottom of the beaker. Separate the copper sulphate crystals from solution by filtration and dry. The soluble impurities present in copper sulphate do not crystallise and hence remain behind in the solution.
Thank you
Well Explained, All the best maam
Well explained,thank you
Thank u mam it helped me in my exams.
thank you . it help in my exam a lot