Contents
Mechanism of Enzyme Catalysed Reactions
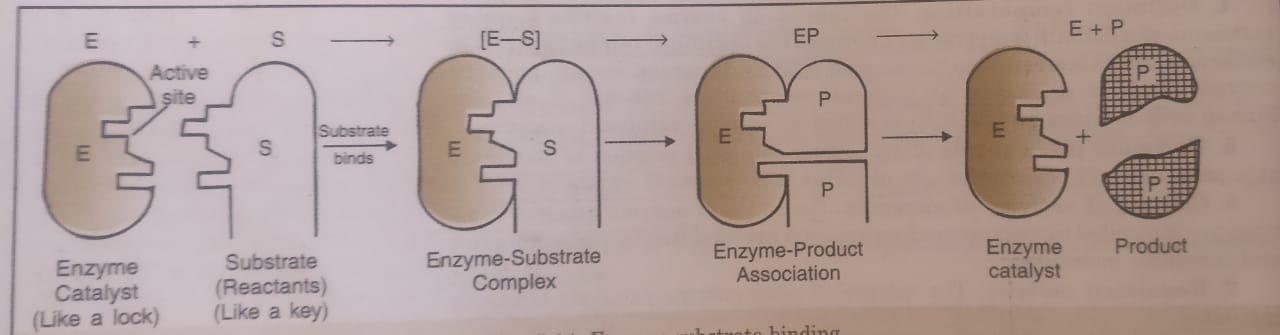
The various steps involved in the enzyme catalysed reaction are given below:
Step 1. Binding of the enzyme (E) to substrate (S) to form enzyme-substrate complex.
E + S ⇔ ES (fast, reversible)
ES is called the enzyme-substrate complex.
Step 2. Dissociation of enzyme-substrate complex to form the products.
ES ——-> EP ——> E —–> P
ES = Enzyme substrate complex
EP = Enzyme product association
E = Enzyme
P= Product
The rate of enzyme catalysed reaction depends upon the concentration of ES. It changes from first order to zero order an the concentration of substrate is increased. The catalytic property of enzymes is present at certain specific regions on their surfaces. These are
called active sites or catalytic sites.
The active sites have characteristic shape and poses active groups such as -NH2 , -COOH , -OH, -SH etc. These molecules of the reactants (substrate) which have complementary shapes fit suitably into these active sites. Specific binding accounts for the high specificity of these enzyme reactions. The specificity of fitting together of the substrate structure and the enzyme structure is explained on the basis of two models:
1 Lock-and-key model.
2 Induced fit model.
lock and key model
The substrate, the molecule on which the enzyme acts, fits into the slot as key fits into a lock. The shape of the active site of any given enzyme is such that only a specific substrate can fit into it, in the same way as one key can open a particular lock.
Unlike an ordinary lock, the protein molecule (enzyme) slightly changes the shape when the substrate lands at the active site. The ability of the enzyme to undergo the correct distortion also determines whether the key will fit or not.
Induced fit model
According to this model substrate induces the active site to adopt a perfect fit rather than a rigidly shaped lock and key. We can picture this model as hand in a glove, in which the glove (active site) does not attain its functional shape until the hand (substrate) moves into place.
Fig. Enzyme Substrate Binding
Applications of Enzymes
1) Industrial applications
The enzymes are widely used in industrial processes.
For example: Enzymes are used in
a) breweries for the manufacture of beer, wine, etc. by the fermentation of carbohydrates.
b) in food processing industries for preparing sweet syrup, etc.
c) in the production of cheese by coagulation of milk.
2) Enzyme deficiencies and prevention of diseases
a) The deficiency of phenylalanine hydroxylase enzyme causes a congenital disease called phenylketone urea. This disease causes accumulation of compounds in the body which results into severe brain damage and retardation in children.
b) Deficiency of enzyme tyrosinase causes albinism.
3) Curing diseases
Certain enzymes are also useful for treating heart diseases. An enzyme streptokinase is used to dissolve blood clot.
Shape selective Catalysis by Zeolites
The catalytic reaction which depends upon the pore structure of the catalyst and the size of the reactant and product molecules is called shape selective catalysis.
Zeolites are good shape selective catalysts because of their honey comb like structures.
1) These are microporous aluminosilicates of the general formula Mx/n[(AlO2)x(SiO2)y]mH2O.
2) These are three dimensional networks of silicates in which some siilcon atoms are replaced by aluminium atoms.
3) These are porous and have cavities of molecular dimensions. The internal structure of a zeolite is a network of tunnels and cavities. Therefore, zeolites have an enormous surface area which is largely on the inside of the solid.
4) The zeolites can permit the entry and exit of molecules of a certain size into the active regions within the holes.
5) These are used in petrochemical industries for cracking of hydrocarbons and isomerization.
6) The reactions in zeolites depend upon the size of the cavities (cages) and pores (tunnels) present them. Zeolite catalysis is the shape selectivity.
7)The pore size in zeolites generally varies between 260 pm and 740 pm. Depending upon the size of the molecules of reactants and products and sizes of the pores of zeolites, reactions proceed in specific manner.
For example: zeolite catalyst known as ZSM-5 converts alcohols to gasoline.The alcohol is dehydrated in the cavities and the hydrocarbons are formed.
To increase the rates of reactions and to get maximum yields of products in minimum time, catalysts are used in the chemical industries.
Important Industrial Catalytic Processes
1) Haber’s process for the manufacture of ammonia
Catalyst : Finely divided iron
Promoter : Molybdenum
Conditions : 200 bar , 723-773 K
N2 (g) + H2 (g) ———> 2 NH3 (g)
2) Ostwald’s process for the manufacture of nitric acid
Catayst: Platinised asbestos
Condition: 573 K
4 NH3 (g) + 5 O2 (g) ——-> 4 NO (g) + 6 H2 (g)
2 NO (g) + O2 (g) —–> 2 NO2 (g)
4 NO2 (g) + 2 H2O(l) + O2 (g) ——> 4 HNO3 (aq)
Leave a Reply