Contents
Colloidal State
Certain solutes such as starch, glue, gelatin etc. could not pass through the parchment membrane while the ordinary solutes such as sodium chloride, urea , sugar etc. can easily do so. Thus, colloid is not a substance but it is a state of a substance which depends upon the molecular size.
Three Types of Solutions
On the basis of particle size of the substance, the solutions may be divided into three types. These are :
1) True solutions
2) Suspensions
3) Colloidal solutions.
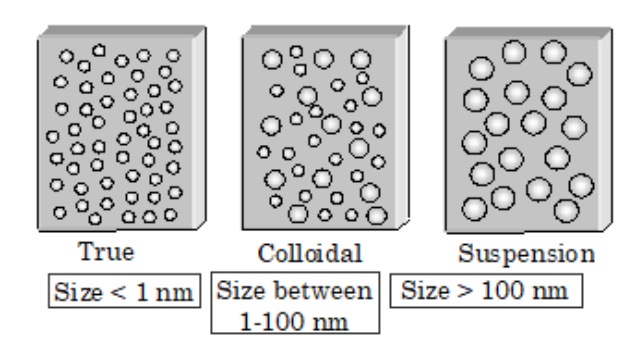
1) True solution is a homogeneous solution which contains small solute particles (molecules or ions) dispersed throughout a solvent.
For example : The solution of sodium chloride in water. The particle size is less than 1 nm. The particles of a solute in a true solution are invisible even under microscope and as particles can pass through ordinary filter paper as well as through animal membrane.
2) Suspension is a heterogeneous mixture which contains small insoluble particles. The particle size is more that 1000 nm.
For example: Dirt particles in water.
The particles of a suspension may not be visible to the naked eye but are visible under a microscope. The particles of a suspension can neither pass through an ordinary filter paper nor through animal membrane.
3) Colloidal solution is a heterogeneous solution which contains particles of intermediate size.
For example: Milk.
a) The particles of a colloidal solution have diameters between 1 to 1000 nm.
b) Such particles cannot be normally seen with naked eye.
c) light reflected by them can be seen under an ultramicroscope.
d) The particles of a colloidal solution can pass through ordinary filter paper but not through animal membrane.
e) In a colloid, the dispersed phase may consist of particles of a single macro molecule (such as synthetic polymer or protein) or an aggregate of many atoms, molecules or ions.
f) Colloidal particles have an enormous surface area per unit mass.
The size of dispersed particles in colloidal solutions is more than that of solute particles in a true solution and smaller than that a suspension.
Phases of colloids and their Classification
Colloidal solution is of heterogeneous nature and consists of two phases i.e. a dispersed phase and a dispersion medium.
1) Dispersed phase
It is the component present in small proportion and is just like a solute in a solution.
For example: In the colloidal solution of silver in water, the silver acts as a dispersed phases.
2) Dispersion medium
It is generally component present in excess and is just like a solvent in a solution.
| Property | True solution | Colloidal Solution | Suspension |
| Size of Particles | Less than 10-9 m | Between range of 10-9 – 10-6 m | More than 10-6 m |
| Nature | Homogeneous | homogeneous but is heterogeneous. | Homogeneous |
| Visibility | The solute particles are invisible to naked eye and also under the microscope. | The solute particles are invisible to naked eye but can be viewed under powerful microscope | The solute particles are visible to naked eye. |
| Stability | The solute particles do not settle down and are stable. | The solute particles do not settle down and are stable. (On centrifugation the particles can settle) | The solute particles settle down and are unstable. |
| Filtration | The solute particles can pass through filter paper and no residue is seen on filter paper. | The solute particles passes through filter paper and no residue is left. (But the particles cannot pass through parchment membrane) | The solute particles can not pass through filter paper and residue is collected on filter paper. |
| Transparency | Transparent | Translucent | Opaque |
Leave a Reply