The surface of a solid has a tendency to attract and retain the molecules of the phase with which it comes into contact. These molecules remain only at the surface of the solid and do not penetrate into the bulk.
For example: When a small amount of finely divided charcoal is put into vessel containing a gas, it is observed that the pressure of the gas decreases rapidly at first and then gradually. The decrease in pressure of the gas is due to the accumulation of the gas on the surface of charcoal. The gas molecules remain only on the surface and do not penetrate into the bulk of the solid. Since these molecules remain only at the surface and do not go deeper into the bulk, their concentration is more at the surface than in the bulk of the solid.
Contents
Adsorption
The phenomenon of higher concentrations of any species at the surface than in the bulk is called adsorption.
or
The phenomenon of attracting and retaining the molecules of substance at the surface of a solid or a liquid resulting into higher concentration of the molecules on the surface than in the bulk is called adsorption.
Solids can adsorb solute molecules from solutions also.
Adsorbent and Adsorbate
The solid substance on the surface of which adsorption occurs is known as adsorbent.
The substances that get adsorbed on the solid surface due to intermolecular attractions are called adsorbate.
The process of removal of an adsorbed substance from the surface on which it is adsorbed is called desorption. It is the reverse of adsorption and can be brought about by heating or by reducing the pressure.
The adsorbent may be a solid or a liquid and the adsorbate may be a gas or a solute in some solution.
Examples of Adsorption
1) Adsorption of a gas by charcoal: Finely divided activated charcoal has a tendency to adsorb a number of gases like ammonia, sulphur dioxide, chlorine, oxygen, hydrogen, carbon monoxide, phosgene, etc. Charcoal acts as an adsorbent while gas molecules act as adsorbate.
Solids particularly, finely divided have a large surface area and therefore, they show the property of adsorption to a much larger extent.
For example: charcoal, silica gel, alumina gel, clay, colloids, metals in finely divided state, etc. are highly good adsorbents because they have highly porous structures and hence large surface area.
2) Adsorption of a dye by charcoal: When animal charcoal is shaken with a solution of an organic dye such as methylene blue it is observed that the solution turns colourless. The discharge of the colour is due to the fact that the coloured component gets adsorbed on the surface of animal charcoal.
3) When aqueous solution of raw sugar is passed over beds of animal charcoal, it becomes colourless because the colouring substances are adsorbed by the charcoal.
4) The air becomes dry in the presence of silica gel because the water molecules get adsorbed on the silica gel.
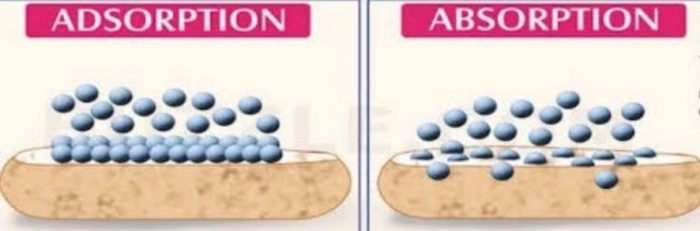
Difference between Adsorption and Absorption
Adsorption is a phenomenon in which there is higher concentration of another substance on the surface than in the bulk.
Absorption is a phenomenon in which the molecules of a substance are uniformly distributed throughout the body of other substance.
a) When a stick of chalk is dipped in ink, the surface retains the colour of ink due to adsorption of coloured molecules. On the other hand, the solvent of the ink goes deeper into the stick due to absorption. When chalk stick is broken, it is found to be white from inside.
b) When silica gel is placed in the environment of water it adsorbs the water vapour. The water vapours are present in high concentration at the surface of silica gel.
c) When anhydrous calcium chloride is placed in the environment of water, it absorbs water. The water vapours uniformly get distributed throughout the body of calcium chloride. Thus, silica gel adsorbs water vapour while anhydrous calcium chloride absorbs water.
| Absorption | Adsorption |
| It is the phenomenon in which the particles of gas or liquid get uniformly distributed throughout the body of the solid. | It is the phenomenon of higher concentration of particles of gas or on the surface than in the bulk of the solid. |
| The concentration is the same throughout the material. Therefore, it is a bulk phenomenon. | The concentration on the surface of the adsorbent is different from that in the bulk. Therefore it is a surface phenomenon. |
| Absorption occurs at uniform rate. | Adsorption is rapid in the beginning and its rate slowly decreases. |
Positive and Negative Adsorption
Positive adsorption
When the concentration of adsorbate is more on the surface of adsorbent relative to its concentration in the bulk, it is called positive adsorption.
Negative adsorption
When the concentration of the adsorbate is less on the surface relative to its concentration in the bulk, it is called negative adsorption.
For example: When a concentrated solution of potassium chloride (KCl) is shaken with blood charcoal: it shows positive adsorption but with a dilute solution of KCl, it shows negative adsorption.
It’s very nice of you to give study’s access to those who are underpriviledged .. God bless you
There’s a request.. can you please post other chapters notes of class 12
very helpful clear cut explanation …thank you so much