Contents
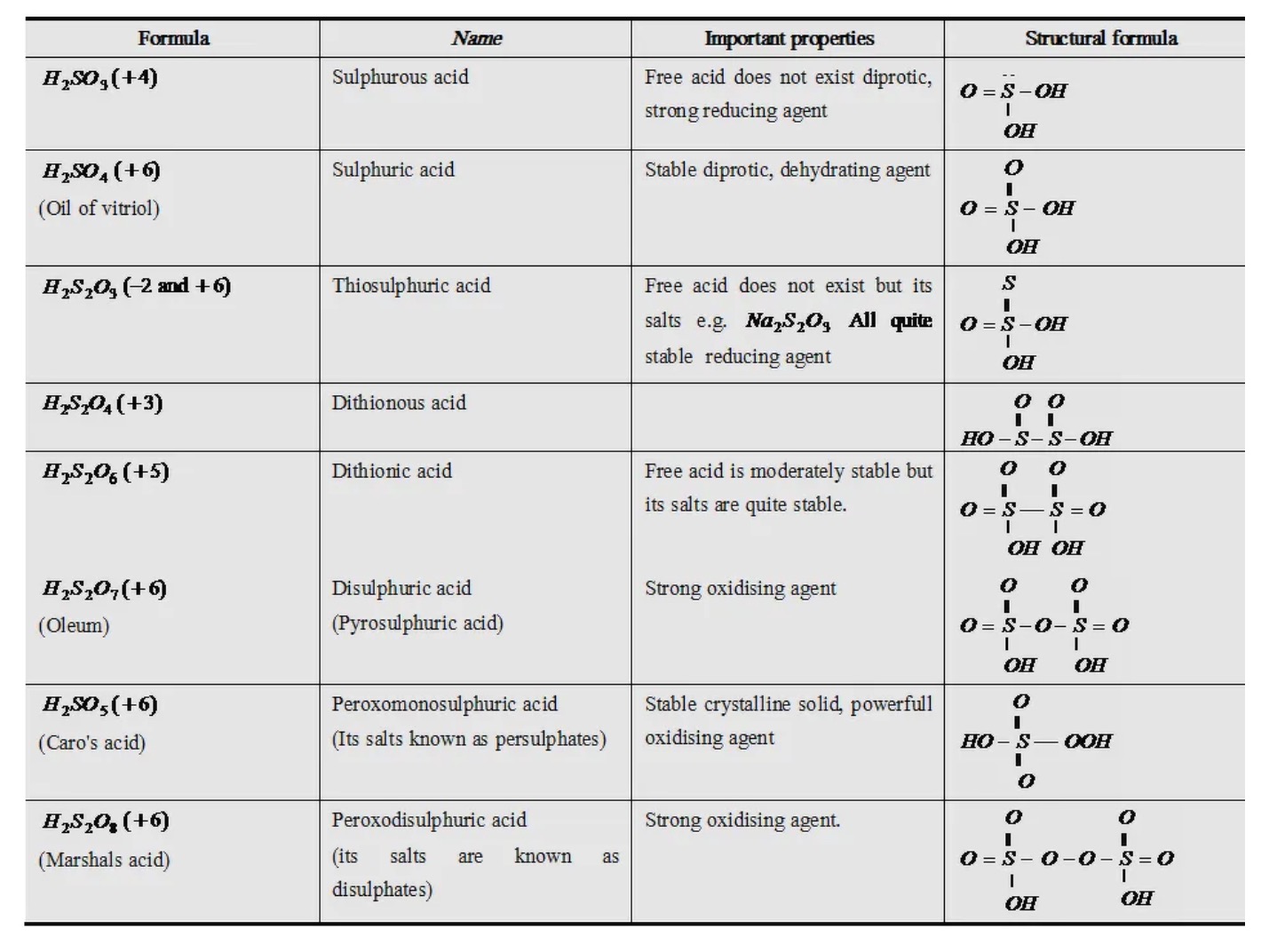
Oxoacids of Sulphur
Sulphuric Acid
Manufacture of Sulphuric Acid
Sulphuric acid can be manufactured by Contact process. The process involves the following steps:
(i) Preparation of sulphur dioxide
Sulphur dioxide is prepared by burning sulphur or iron pyrites in excess of air.
S+O2 → SO2
4FeS2 + 11O2 → 2Fe2O3 + 8SO2
Iron pyrites
(ii) Oxidation of sulphur dioxide into sulphur trioxide
Sulphur dioxide is catalytically oxidised to sulphur trioxide with atmospheric oxygen. The reaction is reversible as well as exothermic in nature. The high yield of SO3 will lead to more production of the acid.
2SO2 (g) + 02 (g) → 2SO3 (g)
ΔrH =-196.6 kJ
(iii) Absorption of sulphur trioxide into 98% sulphuric acid to form oleum
Sulphur trioxide is absorbed in about 98% H2SO4 to form oleum or fuming sulphuric acid.
SO2 + H2SO4 → H2S2O7
Oleum
(iv) Dilution of oleum with water
Oleum is then diluted with required quantity of water to get sulphuric acid of any desired concentration.
2H2S4O7 + H2O → H2SO4
Conditions favouring the maximum yield of sulphur trioxide
The key step in the manufacture of sulphuric acid is the catalytic oxidation of SO2 with O2 to give SO3. The conditions for the maximum yield of sulphur trioxide are derived by using Le Chatelier’s principle as follows:
(i) Low temperature: The forward reaction is exothermic and therefore low temperature favours the oxidation of sulphur dioxide. However, it is essential to have minimum temperature of 720 K, called optimum temperature, to get the maximum yield of the product.
(ii) High pressure: Since the volume of the gaseous products is less than that of the gaseous reactants, high pressure should favour the oxidation of sulphur dioxide. But a very high pressure may cause the corrosion of the vessel in which oxidation is carried. Therefore, a pressure of 2 to 3 bar is sufficient for the oxidation.
(iii) Use of catalyst: A catalyst increases the speed of reaction. Platinised asbestos was used as catalyst. But it is easily poisoned by the impurities present in the gases and therefore, has now been replaced by vanadium pentoxide (V2O5). It is comparatively cheap and is not poisoned by the impurities.
(iv) Purity of gases: The gases must be purified before subjecting them to oxidation in the presence of catalyst.
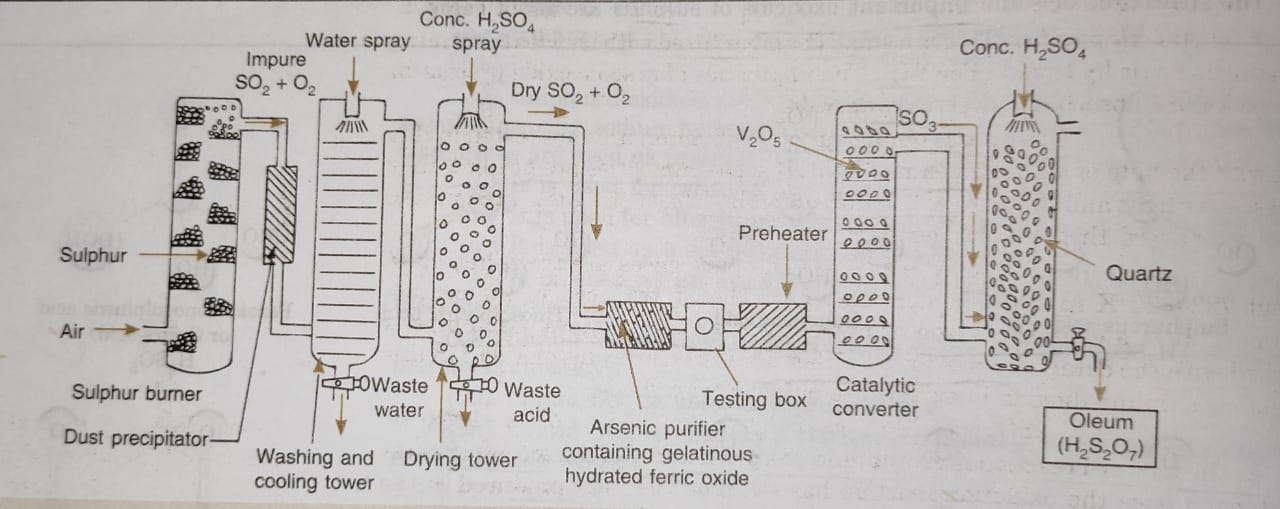
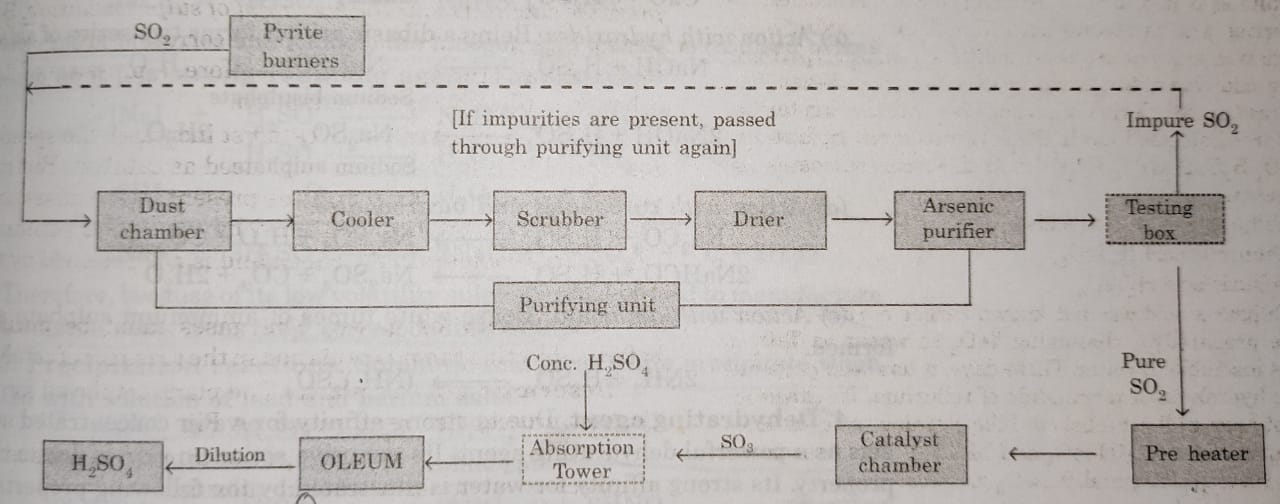
Description of the Sulphuric Acid Plant
(1) Sulphur burners : Sulphur or iron pyrites are burnt in excess of air to form sulphur dioxide.
S + O2 → SO2
4FeS2 + 11O2 → 2Fe2O3 + 8SO2
(2) Purification Unit : The gaseous mixture coming out of sulphur burners is generally impure. The gases are purified as follows :
(i) Dust chamber: Steam is introduced to remove dust particles.
(ii) Coolers: The hot gases are cooled to about 373 K by passing them though cooling pipes.
(iii) Scrubber: Gases are introduced into a washing tower (packed with quartz) also known as scrubber which dissolves mist and any other soluble impurities.
(iv) Drying tower: A spray of conc. H2SO4 is used for drying of gases.
(v)Arsenic purifier: This is a small chamber fitted with shelves containing gelatinous ferric hydroxide Fe(OH)3. The impurities of arsenic oxide present in the gases are absorbed by ferric hydroxide.
(3) Testing box : The gases coming out of purification unit are tested in this box with the help of a strong beam of light. If some impurities are present, they will scatter light and the path will become visible. In case the gases are impure, they are passed through the purifying unit again.
(4) Contact chamber or converter : The pure gases are then, heated to about 723-823 K in a pre-heater. These are then introduced in the contact chamber. It is a cylindrical iron chamber fitted with iron pipes. Each pipe is packed with the catalyst consisting of either platinized asbestos or V2O5. In this chamber sulphur dioxide is oxidised to sulphur trioxide.
2SO2 + O2 → 2SO3 ΔH = -196.6 kJ
As the forward reaction is exothermic, the pre-heating of the incoming gases is stopped once the oxidation reaction has started. The heat produced in the reaction is sufficient to maintain the temperature of the reaction.
(5) Absorption tower: It is a cylindrical tower packed with acid proof flint.Sulphur trioxide escaping from the converter is led to the bottom of the tower while concentrated sulphuric acid (98%) is sparyed from the top. Sulphur trioxide gets absorbed by sulphuric acid to form oleum or fuming sulphuric acid.
H2SO4+ SO3 → H2S2O7
Oleum
Oleum is then diluted with calculated amount of water to get acid of desired concentration.
H2S2O7 + H2O → 2H2SO4
A flow sheet diagram of Contact process is given below:
Physical Properties of Sulphuric Acid
(1) Pure sulphuric acid is a colourless, dense, viscous liquid (specific gravity of 1.84 at 298 K It freezes at 283 K and boils at 611 K.
(2) The high boiling point and viscosity of sulphuric acid suggest that it is an associated molecule.
(3) The different molecules are held together by hydrogen bonding as shown below :
(4) The concentrated acid has strong affinity for water and the dissolution process is highly exothermic and a large quantity of heat is evolved.
(5) Concentrated sulphuric acid is diluted by adding acid to water and not water to acid. In the latter case, so much heat is produced that acid will spurt out of the container.
Chemical Properties of Sulphuric Acid
(1) Dissociation
Sulphuric acid is quite stable but on strong heating, it dissociates into SO3 and H2O.
H2SO4 → H2O + SO3
(2) Acidic character
It is a strong dibasic acid and ionises in aqueous solution as
H2SO4(aq) ⇔ H+(aq) + HSO4¯ (aq) Ka1 = Very large
HSO4¯(aq) ⇔ 2 H+ (aq) + SO42- Ka2 = 1.2 x 10-2
The larger value of Ka1 (Ka1 > 10) means that H2SO4 is largely dissociated into H+ and HSO4¯ ions. Greater the value of dissociation constant (Ka), the stronger is the acid.
Therefore, it forms two series of salts : normal sulphates such as sodium sulphate (Na2SO4), copper sulphate (Cu2SO4), etc. and acid sulphates or hydrogen sulphates or bisulphates such as sodium hydrogen sulphate (NaHSO4). Thus, it reacts with metals, oxides, carbonates, etc. which are the characteristic reactions of an acid.)
(a) Action with metals: Dilute acid, with a greater degree of ionisation, liberates hydrogen with metals like magnesium, zinc, iron, tin, etc.
H2SO4 (dil.) + Zn → ZnSO4 + H2 ↑
H2SO4 (dil.) + Mg → MgSO4 + H2 ↑
However, metals like copper and silver which are less electropositive than hydrogen, fail to react with the dilute acid.
(b) Action with metal oxides
CaO + H2SO4 → CaSO4 + H2O
(c) Action with hydroxides
Being a dibasic acid, it forms two series of salts.
NaOH + H2SO4 → NaHSO4 + H2O
Sodium bisulphate
2NaOH + H2SO4 → Na2SO4 + 2H2O
Sodium sulphate
(d) Action with carbonates and bicarbonates.
Na2CO3 + H2SO4 → Na2SO4 + H2O + CO2
2NAHCO3 + H2SO4 → Na2SO4 + CO2 + 2 H2O
(e) Action with ammonia
Dense white fumes of ammonium sulphate are formed.
2NH3 + H2SO4 → (NH4)2SO4
(3) Dehydrating Agent
Due to strong affinity for water concentrated acid acts as a powerful dehydrating agent. Its corrosive action on skin is due to this property.
(a) Drying of gases : Many wet gases like carbon dioxide, sulphur dioxide, chlorine, hydrogen chloride, etc. which have no action with acid, are dried by bubbling into the concentrated sulphuric acid.
(b) Charring: When concentrated acid is dropped on paper wood or sugar, it absorbs moisture and brings about charring.
C12H22O11 → 11H2O + 12 C
(c) Action with formic acid
HCOOH → CO + H2O
(d) Action with oxalic acid
(COOH)2 → CO + CO2 + H2O
(e) Action with ethyl alcohol
At 170°C, ethyl alcohol gets dehydrated to ethylene.
C2H5OH → C2H4 + H2O
Ethylene
(f) Action with hydrated salts
It removes water of crystallisation from hydrated salts.
For example:
CuSO4.5H2O → CuSO4 + 5 H2O
Hydrated copper Anhydrous copper sulphate (white)
sulphate (blue)
(4) Action on metals
Concentrated sulphuric acid reacts with metals like zinc, silver, copper, mercury, lead, etc. upon heating to form sulphur dioxide.This is due to oxidising property of the acid.
(5) Action with salts
It is a strong non-volatile acid and decomposes the salts of volatic acids forming its own salts.
2MX + H2SO4 → 2 HX + M2SO4 (M = metal, X = F, CI, NO3)
Na2CO3 + H2SO4 → Na2SO4 + H2O + CO2
KNO3 + H2SO4 → KHSO4 + HNO3
NaCl + H2SO4 → NaHSO4 + HCl
In case of bromides and iodides, the halogen acid formed is oxidised to free halogen as it is a strong reducing agent.
For example:
NaI + H2SO4 → NaHSO4 + HI ] × 2
H2SO4 → H2O + SO2 + O
2HI + O → H2O + I2
—————————————————————-
2NaI + 3H2SO4 → 2NaHSO4 + SO2 + I2 + 2 H2O
—————————————————————-
Therefore, because of its low volatility sulphuric acid is used to manufacture more volatile acids from their corresponding salts.
(6) Precipitation reactions
Sulphuric acid gives white precipitate when treated with solution of lead and barium salts.
BaCl2 + H2SO4 → BaSO4 + 2HCl
Pb(NO3)2 + H2SO4 → PbSO4 + 2 HNO3
(CH3COO)2Pb + H2SO4 → PbSO4 + 2CH3COOH
(7) Action with sulphur trioxide
Sulphur trioxide is absorbed by conc. sulphuric acid to form pyrosulphuric acid known as oleum or fuming sulphuric acid.
H2SO4 + SO3 → H2S2O7
(8) Oxidising agent
Hot concentrated sulphuric acid is a moderately strong oxidising agent. The oxidising character of H2SO4 is due to the fact that it decomposes to give nascent oxygen.
H2SO4 → H2O + SO2 + [O]
The nascent oxygen brings about a number of oxidation reactions. Both metals and non-metals are oxidised by concentrated sulphuric acid which is reduced to SO2
(a) It oxidises carbon to carbon dioxide.
2H2SO4 + C → 2SO2 + CO2+ 2H2O
(b) It oxidises sulphur to sulphur dioxide.
2H2SO4 + 3S → 3 SO2 + 2H2O
(c) It oxidises phosphorus to phosphoric acid.
10 H2SO4 + P4 → 4H3PO4 + 10 SO2 + 4H2O
(d) It oxidises hydrogen sulphide to sulphur.
H2SO4 + H2S → 2H2O + SO2 + S
(e) It oxidises halogen acids (HBr and HI) to halogens.
H2SO4 + HBr → 2H2O + Br2 + SO2
(f) It oxidises metals such as Cu, Pb, Hg, Ag etc. to their corresponding sulphates liberating SO2.
Cu + 2H2SO4 → CuSO4 + SO2+ 2H2O
Uses of Sulphuric Acid
(i) In fertilizer industry: It is used in the preparation of fertilizers such as ammonium phosphate, ammonium sulphate, super phosphate of lime, etc.
(ii) In petroleum refining: It is used for the refining of crude petroleum.The crude petroleum is treated with sulphuric acid to remove unwanted sulphur and other tarry compounds.
(iii) In dyes, drugs, paints and pigments. It is used directly or indirectly in the manufacture of chemicals such as dyes, drugs, paints, pigments, etc.
(iv) In chemical industry: It is used for the manufacture of hundreds of other compounds such as hydrochloric acid, nitric acid, phosphoric acid, sulphates, bisulphates, diethyl ether, etc.
(v) In metallurgy: Sulphuric acid is used for metallurgical processes such as electrolytic refining, electroplating, galvanising, etc. A number of metals like copper, silver, etc. are extracted from their ores using sulphuric acid.
(vi) It is used for cleaning the surfaces of metals (picking) before electroplating.
(vii) It is used in the manufacture of explosive such as dynamite, T.N.T. nitro cellulose products (gum, cotton), etc.
(viii) It is also used as a drying and dehydrating agent.
(ix) It is used for storage batteries.
(x) As a laboratory reagent: It is widely used in laboratory as a drying and dehydrating agent and in many other chemical reactions. It is very important chemical reagent.
Leave a Reply