Contents
Group 18
Occurrence of Noble Gases
Due to the inert nature of noble gases, they always occur in the free state. Except radon, all these gases are present in atmosphere in the atomic state. Their total percentage in dry air is about 1% by volume, of which argon (0.93%) in the major component. It originates in the air mostly from electron capture of potassium.
Helium is also present in natural gas to the extent of 2 to 7%. Helium and sometimes neon are found in small quantities in minerals of radioactive element such as monazite, clevite, pitch blende, etc. Helium, neon and argon are found in the water of certain springs.
Radon is radioactive and does not occur in the free state because it decays very rapidly.
General Characteristics of Group 18 Elements
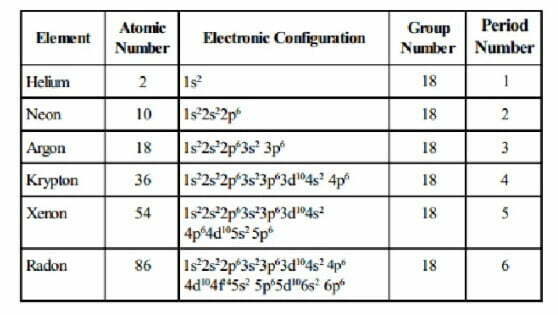
(1) Electronic Configurations
Except helium, the atoms of all noble gases have eight electrons in the valence shell. The general electronic configuration of noble gases (except He) may be expressed as ns2np6. On the other hand, helium has 1s2 electronic configuration.
These configurations being stable, the noble gases neither have any tendency to gain nor lose electrons, and, therefore, they do not enter into chemical combinations. It is, therefore, reasonable to assume that inert nature of the noble gases is due to their stable electronic configurations.
(2) Atomic and Physical Properties
(a) Existence
(b) Atomic radii
(c) Ionisation Enthalpies
The ionisation enthalpies of noble gases are very high. This is attributed to the stable completely filled configurations of noble gases. However, the ionisation enthalpies decrease with increase in atomic number from He to Rn due to increasing atomic size.
(d) Electron Gain Enthalpies
Due to the stable ns2np6 electronic configurations, noble gas atoms have no tendency to accept additional electron. Therefore, their electron gain enthalpies are zero or have large positive values.
(e) Melting and Boiling Points
These van der Waal’s forces increase with the increase in atomic size of the atom, and therefore, the boiling points and melting points increase from He to Rn. Helium has the lowest boiling point (4.2 K) of any known substance.
(f) Ease of liquefaction
The noble gases are not easily liquefied. This is due to the fact that there are only weak van der Waal’s forces which hold atoms together.
Due to increase in atomic size and, therefore, increase in van der Waal forces, the ease of liquefaction increases down the group from He to Rn.
(g) Solubility in water
Properties of Noble Gases
Physical Properties
(ii) All the noble gases are monoatomic.(iii) They are sparingly soluble in water.
Chemical Properties
(i) The noble gases have completely filled ns2np6 electronic configurations in their valence shells.
(ii) The noble gases have very high ionisation energies.
(iii) The electron affinities of noble gases are almost zero or large and positive.
Therefore, they have neither tendency to gain nor to lose any electron and do not enter into chemical combinations.
In March 1962, Neil Bartlett noticed that platinum hexafluoride, PtF6 is a powerful oxidising agent which combines with molecular oxygen to form red ionic compound, dioxygenyl hexafluoroplatinate (V), O2+ [PtF6]–
Oxygen and xenon have some similarities:
(i) The first ionisation energy of xenon gas (1170 kJ mol-1) is fairly close to that of oxygen (1175 kJ mol-1).
(ii) The molecular diameter of oxygen and atomic radius of xenon are similar (4A°).
On this assumption, Bartlett reacted xenon and platinum hexafluoride in gas phase and an orange yellow solid of the composition XePtF6 was obtained.
The formation of this compound has shown that xenon is not totally inert and after this a number of compounds of xenon were prepared.
Leave a Reply