Phosphorus and Phosphine – The p-Block Elements – Class 12
Contents
Phosphorus
Phosphorus is produced by heating bone ash or phosphate rock with silica (SiO2) and coke in an electric furnace at 1775 K.
The reactions taking place are :
2Ca3(PO4)2 + 6SiO2 ——-> 6CaSiO3 + P4O10
P4O10 +10C ——-> 10 CO + P4
The vapour of phosphorus thus produced upon condensation give white phosphorus, which exists as P4.
Allotropic Forms of Phosphorus
Phosphorus exists in many allotropic forms. Of these three main allotropic forms are :
(i) White Phosphorus
(ii) Red Phosphorus
(iii) Black Phosphorus
(1) White Phosphorus
a) It is obtained from phosphorite rock with coke and sand in an electric furnace at 1775 K.
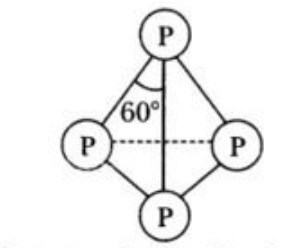
b) It consists of P4 units. In this case, the four P-atoms lie at the corners of a regular tetrahedron with angle PPP= 60°.
c) Each phosphorus is bonded to each of the other three P-atoms by covalent bonds, so that each P-atom completes its valence shell.
Properties:
The main characteristics of white phosphorus are :
(i) It is soft, translucent white waxy solid with garlic smell.
(ii) It can be cut with a knife.
(iii) It melts at 317 K and boils at 553 K.
(iv) It is insoluble in water but soluble in benzene, carbon disulphide, liquid NH3.
(v) It is very poisonous. The vapours inhaled, may prove fatal.
(vi) It glows in dark (a property known as chemiluminescence).
(vii) White phosphorus, P4 molecule are stable upto 1070 K even in the vapour phase. When heated above 1070 K, P4 molecules begin to dissociate into P2 molecules which have a bond enthalpy of 489.6 kJ mol-1.
(viii) White phosphorus is less stable and therefore, more reactive than the other solid phases under normal conditions. This is because of angular strain in the P4 molecules where the angles are only 60°. Its ignition temperature is very low (303 K) and therefore, it catches fire in air to form dense white fumes of P4O10. Therefore, it cannot be kept in air to form dense white fumes of P4O10.It is generally stored under water.
P4 + 5O2 —-> P4O10
(ix) It combines with metals forming their phosphides such as Na3P, Mg3P2, Ca3P2, Ag3P, Cu3P2 etc.
12Na + P4 —> 4 Na3P
Sodium phosphide
4Ag + P4 —> 4 Ag3P
Silver phosphide
6 Mg + P4 —> 2 Mg3P2
Magnesium phosphide
(x) It is a weak reducing agent and reduces sulphuric acid to sulphur dioxide, nitric acid to nitrogen peroxide, etc.
P4+10H2SO4 ——> 10SO2 + 4H20 + 4H3PO4
Phosphoric acid
P4+ 20 HNO3 ——> 4H3PO4 + 20 NO2 + 3H20
It also reduces solutions of copper, silver and gold salts to their corresponding metals.
P4 +3CuSO4 + 6H2O —-> Cu3P2 + 2H3PO3 + 3H2SO4
Cu3P2 + 5CuSO4 + 8H2O —–> 8Cu + 5H2SO4 + 2H3PO3
P4 + 8CuSO4 + 14H2O —–> 8Cu + 8H2SO4 + 2H3PO3 + 2 H3PO4
(xi) It readily combines with halogens to form trihalides (PX3) and on prolonged treatment forms pentahalides (PX5).
For example: white phosphorus catches fire in chlorine forming phosphorus trichloride (PCl3) and phosphorus pentachloride (PCl5).
P4 + 6Cl2 —–> 4 PCl3
P4 + 10 Cl2 ——-> 4 PCl5
(xii) White phosphorus combines with sulphur with explosive violence forming a number of sulphides such as P2S3, P2S5, P4S3, P4S5
8P4 + 3S8 ——> 8P4S3
Tetraphosphorus trisulphide
The sulphide, P4S3 is used in strike anywhere matches.
(xiii) On heating with caustic soda solution, it forms phosphine.
P4 + 3NaOH + 3H2O —> PH3 + NaH2PO2
Phosphine Sodium hypophosphite
(2) Red Phosphorus
It is obtained by heating white phosphorus in an inert atmosphere (out of contact of air) at
573 K for several days.
P4(s) ———————-> P4 (s)
White Phosphorus Red phosphorus
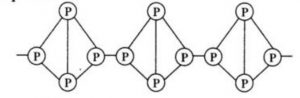
Red phosphorus also exists as P4 tetrahedra but have polymeric structure consisting of
P4 tetrahedra linked together.
The important characteristics of red phosphorus are :
(i) It is a hard crystalline solid without any smell. It possess iron grey lustre.
(ii) It is non-poisonous in nature.
(iii) It is insoluble in water as well as carbon disulphide.
(iv) It is denser than white phosphorus.
(v) It does not glow in the dark.
(vi) Red phosphorus is quite stable and its ignition temperature is quite high (543 K). It, therefore, does not catch fire easily.
(vii) Chemically it is less reactive than white phosphorus.
(viii) It is a bad conductor of electricity.
(ix) It burns with oxygen at 565 K to form phosphorus pentoxide.
P4(s) + 5O2 (g) —–> P2O10(s)
(x) Being less reactive than white phosphorus, it reacts with halogens, sulphur and alkali metals only when heated forming their corresponding salts.
P4 + 6Cl2 —-> 4 PCl3
P4 + 10Cl2 —-> 4 PCl5
P4 + 3S8 —> 8 P2S3
P4 + 12 Na —> 4 Na3P
(xi) Red phosphorus does not react with caustic alkalies. This property is made use in separating red phosphorus from white phosphorus.
(xii) Red phosphorus can be converted into white phosphorus by boiling it in an inert atmosphere and then condensing the vapours of white phosphorus formed under water.
(3) Black Phosphorus
Black phosphorus has two forms; α-black phosphorus and β-black phosphorus, α-black phosphorus is formed by heating red phosphorus in a sealed tube at 803 K.
It can be sublimed in air and has opaque monoclinic or rhombohedral crystals.
β-Black phosphorus is obtained by heating white phosphorus at 473 K under very high pressure (4000-12000 atm) in an inert atmosphere.
White phosphorus ————> α- Black phosphorus (473 K, 4000-1200 atm. pressure )
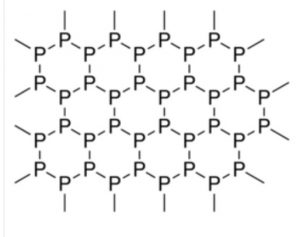
It has a double layered crystal lattice. Each layer is made up of zig-zag chains with P-P-P bond angles of 99° and P-P bond distance of 218 pm. Since it is highly polymeric, therefore, it has high density.
The important characteristics of black phosphorus are:
(i) It has a black metallic lustre.
(ii) It has sharp melting point of 860 K. Its specific gravity is 2.69.
(iii) It has a highly polymeric layered structure and exists in three crystalline and one amorphous forms. Therefore, it has high density.
(iv) It in a moderate conductor of heat and electricity.
(v) It is thermodynamically the most stable and inactive form of phosphorus. It does not burn in air upto 673 K.
Phosphine
Phosphoine is hydride of phophorus, PH3.
Preparation of Phosphine
1) From phosphides: By the action of water or dilute mineral acid on metallic phosphides (Na3P, Ca3P2 ,AlP etc.)
Na3P + 3 H2O ——> 3 NaOH + PH3
Sod. phosphide
Ca3P2 + 6H2O —-> 3Ca(OH)2+ 2PH3
Cal. phosphide
Ca3P2 + 6HCl —–> 3 CaCl2 + 2 PH3
Calcium phosphide
AlP + 3HCl —–> AlCl3 + PH3
Aluminium phosphide
(ii) From phosphorous acid
Pure phosphine can be prepared by heating phosphorous acid at 478-483 K.
4H3PO4 ——–> 3H3PO4 + PH3
Phosphorous acid Phosphoric acid
(iii) From phosphonium salts:
Pure phosphine can also be obtained by heating phosphonium iodide with caustic soda solution.
PH4I + NaOH —–> NaI + H2O + PH3
Phosphonium iodide is obtained from phosphorus.
P4 + 2I2 + 8 H2O —> 2PH4I + 2HI + 2H3PO4
(iv) Laboratory preparation:
Phosphine is prepared in the laboratory by heating white phosphorus with concentrated sodium hydroxide solution in an inert atmosphere of carbon dioxide or coal gas.
4P +3NaOH+ 3H2O —–> NaH2PO2 + PH3
Sodium hypophosphite
Pure phosphine is not inflammable but the gas may catch fire in air. This may be due to the presence of impurity of P2H4 which is spontaneously inflammable. Therefore, a current of carbon dioxide, coal gas is passed through the flask to displace air.
To get pure phosphine, the gas is passed through a U-tube placed in a freezing mixture. The liquid P2H4 gets condensed while PH3, remains unaffected.
Impure gas may also be purified by treating it with hydrogen iodide followed by heating with KOH solution.
PH3 + HI ——-> PH4I
Phosphonium
PH4I + KOH —–> PH3 + KI + H2O
The impurity P2H4 may also be controlled by using alcoholic solution of potassium hydroxide in place of aqueous solution of caustic soda.
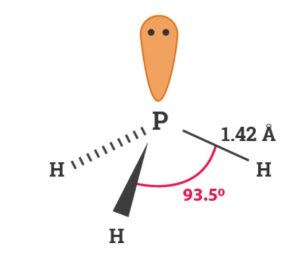
Structure of Phosphine
Like ammonia, phosphine has pyramidal structure. Phosphorus involves sp3 hybridisation. Three bonds are formed by the overlap of sp3 hybrid orbitals of phosphorus with 1s-orbital of hydrogen. One of the orbital is occupied by a lone pair of electrons. The HPH bond angle in PH3 is 93.6° and P-H bond distance 142 pm.
Properties of Phosphine
Physical properties
(i) It is a colourless gas with unpleasant smell of rotten fish or garlic.
(ii) It is highly poisonous in nature.
(iii) It is heavier than air (vapour density = 17) and is slightly soluble in water.
(iv) On cooling to 185.5 K, phosphine condenses to a liquid and on cooling to 139.5 K, it solidifies.
(v) It is slightly soluble in water. The solution of PH, in water decomposes in presence of light giving red phosphorus and hydrogen.
Chemical Properties
1) Action towards litmus
It is feebly basic and reacts with halogen acids to form a phosphonium salt (basic nature).
PH3 + HX —–> PH+4X¯
Phosphonium halide
PH3 + HCl ——> PH4Cl
Phosphonium chloride
PH3 + HBr ——> PH4Br
Phosphonium bromide
PH3 + HI ——> PH4I
Phosphonium iodide
2) Combustibility
Pure phosphine is not combustible. But when heated to 423 K in air or oxygen, it burns forming phosphoric acid.
PH3 + 2O2 —–> H3PO4
3) Decomposition
By heating in the absence of air at 713 K or by passing an electric spark through it, phosphine breaks into its elements.
4PH3 ——–> P4 + 6H2 (713 K , absence of air)
4) Action with chlorine
When heated in the atmosphere of chlorine, phosphine burns forming phosphorus trichloride(PCl3) or pentachloride(PCl5)
PH3 + 3Cl2 —-> PCl3 + 3 HCl
PH3 + 4Cl2 —-> PCl5 + 3HCl
5) Action with nitrous oxide
When sparked with nitrous oxide, it reduces the latter to nitrogen.
PH3+ 4N2O —–> H3PO4 + 4N2
6) Action with nitric oxide
Nitric oxide is reduced to nitrogen.
PH3 + 4NO —–> H3PO4+ 2 N2
7) Precipitation reactions
When bubbled through certain metallic salt solutions, phosphides of the metals get precipitated.
3CuSO4 + + 2PH3 ——> Cu3P2 + 3 H2SO4
Copper sulphate Cupric phosphide
3 HgCl2 + 2PH3 ——> Hg3P2 + 6HCl
Mercuric chloride Mercuric phosphide
3 AgNO3 + PH3 ——> Ag3P + 3HNO3
Silver nitrate Silver phosphide
Using silver phosphide is converted to silver as follows:
Ag3P + 3AgNO3 + 3H2O——-> 6Ag + 3HNO3 + H3PO3
Uses of Phosphine
(i) It is used in preparing Holme’s signals for the ships to know about the position of the rocks in the sea.
(ii) It is used to prepare Smoke screens in warfare. Calcium phosphide reacts with water to form phosphine which burns in air to form P4O10 which acts as a smoke screen.
Leave a Reply