Contents
Ozone
Ozone is an allotropic form of oxygen. It is present in the upper atmosphere (about 20 km above the surface of the earth). It is believed to be formed in the upper atmosphere by the action of ultraviolet rays on oxygen as
3O2 + Ultraviolet rays → 2O3
ΔH298K = 142.7 kJ mol-1
Ultraviolet rays, which are harmful to human beings, are absorbed by oxygen to form ozone. The layer of ozone thus formed also prevents the remaining ultraviolet rays to reach the earth’s surface.
Preparation of Ozone
Ozone is prepared by passing silent electric discharge through pure, cold and dry oxygen in a specially designed apparatus called ozoniser. The formation of ozone from oxygen is an endothermic reaction.
3O2 + Ultraviolet rays → 2O3
ΔH = 142.7 kJ mol-1
Since the formation of ozone is an endothermic process, therefore, it is necessary to use a silent electric discharge (a sparkless electric discharge).The purpose of silent electric discharge (a sparkless electric discharge) is to produce less heat. If concentration of ozone greater than 10% are required, a battery of ozonisers can be used and the pure ozone (b.p. 385 K) can be condensed in a vessel surrounded by liquid oxygen.
Ozone is prepared in the laboratory by the following two types of ozonisers:
(i) Siemen’s ozoniser
(ii) Brodie’s ozoniser
Preparation of pure Ozone
In order to get pure ozone, the ozonised oxygen is cooled by liquid air when ozone (b.p. 3855 K) condenses in preference to oxygen (b.p. 90 K). The liquid thus formed still contains some dissolved oxygen which can be removed by carrying out fractional distillation.
Properties of Ozone
Physical Properties of Ozone
(i) It is pale blue gas having pungent odour.
(ii) It is heavier than air. Its vapour density is 24 while that of air is 14.4. Therefore, it is about 1.5 times heavier than air.
(iii) Its boiling point is 385 K and melting point is 24 K.
(iv) It is slightly soluble in water but more soluble in organic solvents like turpentine oil, carbon tetrachloride, etc.
(v) It liquefies to a deep blue liquid at 161.2 K which can be solidified to a black violet solid at about 80.6 K.
(vi) In contrast to dioxygen which is paramagnetic, ozone is diamagnetic.
(vii) The small concentrations of ozone are harmless. However, if the concentration rises above about 100 ppm, breathing becomes uncomfortable resulting in headache and nausea.
Chemical Properties of Ozone
The important chemical properties of ozone are discussed below :
(1) Action with litmus
Ozone is neutral towards litmus because it does not give any colour change with blue or red litmus.
(2) Decomposition
Ozone is thermodynamically unstable with respect to oxygen because it results in liberation of heat (ΔH is negative) and increase in entropy (ΔS is positive). These two factors reinforce each other resulting in large negative Gibb’s free energy change (ΔG is negative) for its conversion into oxygen. Therefore, the high concentrations of ozone can be dangerously explosive.
On heating above 475 K, it decomposes readily to form oxygen gas :
2O3 → 3O2
ΔH = – 285.4 kJ
The reaction is catalysed by substances like platinum black, manganese dioxide, copper oxide, etc.
(3) Oxidising agent
Ozone is a stronger oxidising agent than molecular oxygen because ozone has higher energy content and decomposes to give atomic oxygen as :
O3 → O2 + O
Therefore, ozone oxidises a number of non-metals and other reducing agents.
For example
(a) Oxidation of compounds
(i) Ozone oxidises black lead sulphide to white lead sulphate :
O3 → O2 + O ]×4
PbS + 4O → PbSO4
————————————
PbS + 4O3 → PbSO4 +4O2
————————————–
Similarly, ozone oxidises sulphides of copper, zinc, cadmium, etc. to their corresponding sulphates.
ZnS + 4O3 → ZnSO4 + 4O2
CuS + 4O3 → CuSO4 + 4O2
CdS + 4O3 → CdSO4 + 4O2
(ii) Ozone oxidises halogen acids to corresponding halogens
O3 → O2 + O
2HCl + O → H2O + Cl2
—————————————
2HCl +O3 → H2O + Cl2 + O2
————————————————
(iii) Ozone oxidises nitrites to nitrates
O3 → O2 + O
KNO2 + O3 —-> KNO3 + O2
————————————–
KNO2 + O3 → KNO3 + O2
—————————————
(iv) Ozone oxidises acidified ferrous salts to ferric salts
O3 → O2 + O
2 FeSO4 + H2SO4 + O —–> Fe2(SO4)3 + H2O
————————————————————
2 FeSO4 + H2SO4 + O3 —–>Fe2(SO4)3 + H2O + O2
————————————————————————–
(v) Ozone oxidises Potassium iodide to iodine
O3 → O2 + O
2kI + H2O + O → 2KOH + I2
———————————————-
2KI+ H2O + O3 → 2KOH + I2 + O2
———————————————–
(vi) Ozone oxidises potassium ferrocyanide to potassium ferricyanide
O3 → O2 + O
2K4[Fe(CN)6] + H2O + O → 2K3[Fe(CN)6] + 2KOH
———————————————————————–
2K4[Fe(CN)6] + H2O + O3 → 2K3[Fe(CN)6] + 2KOH + O2
————————————————————————-
(vii) Ozone oxidises potassium manganate to potassium permanganate
O3 → O2 + O
2K2MnO4 + O + H2O → 2KMnO4 + 2KOH
———————————————————-
2K2MnO4 + O3 + H2O → 2KMnO4 + 2KOH + O2
———————————————————————-
(b) Oxidation of non-metals
(viii) Ozone oxidises moist iodine to iodic acid
O3 → O2 + O ]×5
I2 + 5 (O) → I2O5
I2O5 + H2O → 2 HIO3
———————————————
I2 + 5O3 + + H2O → 2 HIO3 + 5 O2
——————————————————
(ix) Ozone oxidises Sulphur to sulphuric acid
O3 → O2 + O ]×3
S + 3O → SO3
SO3 + H2O → H2SO4
——————————————
S + 3O3 + H2O → H2SO4 + 3O2
——————————————
(x) Ozone oxidises Phosphorus to Phosphoric acid
O3 → O2 + O ]×10
P4 + 10 O → P4O10
P4O10 + 6H2O → 4 H3PO4
—————————————————-
P4 + 10 O3 + 6 H2O → 4 H3PO4 + 10O2
—————————————————-
(c) Oxidation Of metalloids
(i) Oxidation Of moist arsenic
O3 → O2 + O ]×5
2As + 5O → As2O5
As2O5 + 3H2O → 2H3AsO4
————————————————–
2As + 5O3 + 3H2O → 2H3AsO4 + 5O2
————————————————–
(ii) Oxidation of moist antimony
O3 → O2 + O
2Sb + 5O → Sb2O5
Sb2O5 + 3H2O → 2H3SbO4
————————————————–
2Sb + 5O3 + 3 H2O → 2H3SbO4 + 5O2
—————————————————
(d) Oxidation of metals
(i) Silver is oxidised to silver oxide
O3 → O2 + O
2Ag + O → Ag2O
——————————-
2Ag + O3 → Ag2O + O
——————————-
(ii) Mercury is oxidised to mercurous oxide
O3 → O2 + O
2Hg + O → Hg2O
———————————-
2Hg + O3 → Hg2O + O2
———————————–
(4) Bleaching agent
Due to the oxidising action of ozone, it acts as a mild bleaching agent as well as a sterilising agent. It acts as a bleaching agent for vegetable colouring matter.
Vegetable colouring matter + O3 → Oxidised coloured matter + O2
(colourless)
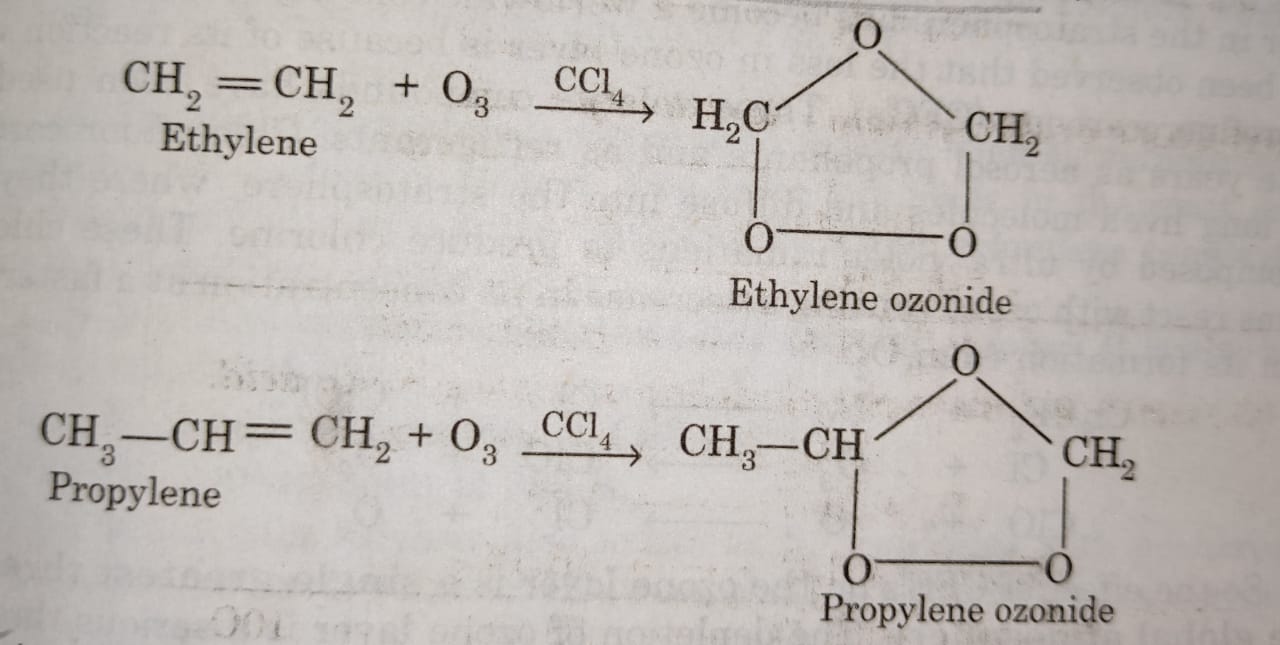
(5) Formation of ozonides
Ozone reacts with alkenes in the presence carbon tetrachloride (CCl4) to form an ozonide.
For example:
(6) Action with peroxides
Ozone reacts with peroxides such as hydrogen peroxides and barium peroxide and liberates oxygen.
H2O2 + O3 → H2O + 2O2
Ba2O2 + O3 → BaO + 2O2
Structure of Ozone
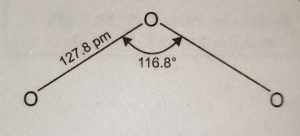
(1) The structure of ozone molecule is angular.
(2) The O-O-O bond angle is 116.8° and 0-0 bond length is 127.8 pm.
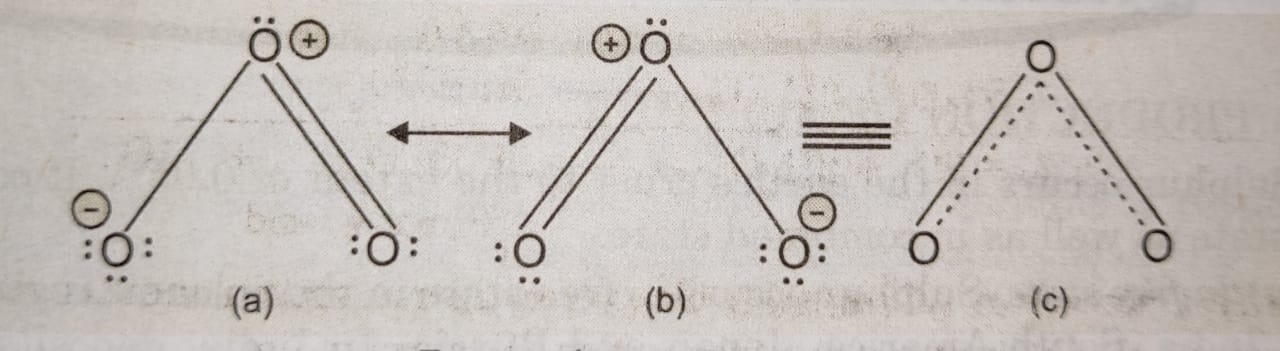
(3) The bond length in ozone molecule is intermediate between single and double bonds in oxygen atoms (single 0-0 bond = 148 pm, double 0=0 bond = 122 pm). Ozone molecule thus, is considered to be a resonance hybrid of the following two resonating structures (a) and (b).
Uses of Ozone
(1) Ozone is used for disinfecting and sterilising water because ozone has germicidal properties.
(2) It is used for bleaching, flour delicate fabrics, oils, flour, starch, ivory, etc.
(3) It is used for purifying air of crowded places such as cinemas, underground railways, auditoriums, tunnels, mines, etc. and for destroying objectionable odours in slaughter houses.
(4) It is used in industry for the manufacture of potassium permanganate, artificial silk, synthetic camphor etc.
(5) It is used in the laboratory for the ozonolysis of organic compounds.
Leave a Reply