Contents
Oxoacids of Halogens
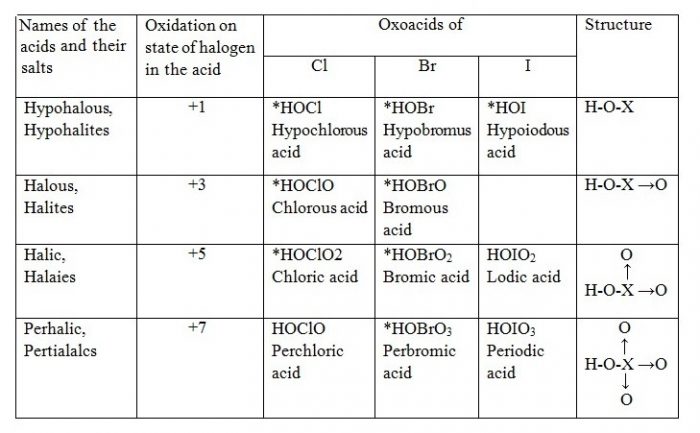
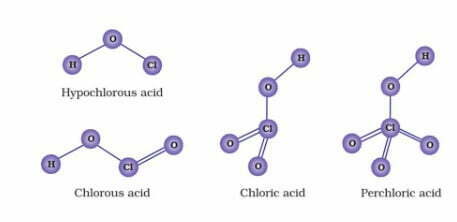
Acidic Character of Oxoacids of Halogens
| Acid | HClO | HBrO | HIO |
| Ka | 2.9 × 10-8 | 5 × 10-9 | 10-11 |
Explanation
HClO < HClO2 < HClO3 < HClO4
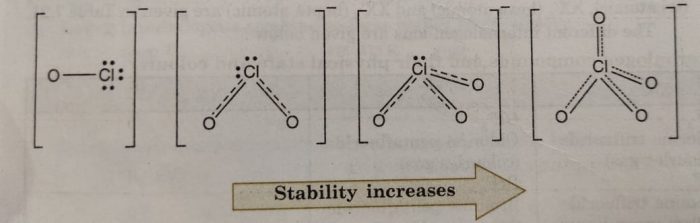
Let us consider the stabilities of the conjugate bases, ClO¯, ClO2¯, ClO3‾, and ClO4¯ formed from these acids, HClO, HClO2, HClO3 and HClO4 respectively.
These anions are stabilized by the delocalisation of the charge between oxygen atoms. If the ion is stabilized to greater extent, it has lesser attraction for the proton and therefore, will behave as weaker base (lesser tendency for the reaction to go in backward direction). Consequently, the corresponding acid will be strong because weak conjugate base has strong acid and strong conjugate base has weak acid and vice versa. Now, the charge stabilization is minimum in ClO¯ and maximum in ClO4¯. The charge stabilization increases in the order:
This means that ClO¯ will have minimum stability and therefore, will have maximum attraction for the H+. In other words, ClO¯ will be strongest base and so its conjugate acid HClO will be the weakest acid.
Similarly, in this series, ClO4¯ is the weakest base (maximum stabilized) and its conjugate acid HClO4, is the strongest acid. Thus, the acidic strength increases in the order.
HClO < HClO2 < HClO3 <HClO4
Interhalogen Compounds
The compounds containing two or more halogen atoms are called interhalogen compounds. They may be represented by the general formula XXn’ , where X is a halogen of larger size and X’ of smaller size and X is more electronegative than X’.
(i) Neutral interhalogens
(ii) Interhalogen anions and cations
Nomenclature
Preparation
KI + 4F2 → KF + IF7
(i) Polar Nature
(ii) Physical State
(iii) Reactivity
2M + XX’ → MX + MX’
2Na + ICI → NaI + NaCl
(iv) Fluorinating Agents
Most of the interhalogens containing fluorine act as powerful fluorinating agents. They convert several metals into their oxides and chlorides to fluorides.
W + 6ClF → WF6 + 3Cl2
2AgCl + ClF3 → 2AgF + Cl2 + ClF
CIF3 or BrF3 are used for the production of UF6 in the enrichment of uranium (236U).
(v) Hydrolysis
All the interhalogen compounds undergo hydrolysis giving halide ion (derived from the smaller halogen atom) and a hypohalite (for XX’), halite (for XX3’), halate (for XX5‘) and perhalate (for XX7‘) anion derived from the larger halogen.
XX’ + H2O → HX’ + HOX
ICI + H2O → HCl + HOI
BrF5 + H2O → 5HF + HBrO3
(v) Ionisation
Interhalogen compounds are partially ionised in the solution or in the liquid state.
For example: Fused ICI and ICI3 have specific conductance of the order of 10 ohm-3 cm-1
2ICI ⇔ I++ ICl2¯
2ICl3 ⇔ ICl2+ + ICI4¯
(vi) Oxidising Agents
The interhalogen compounds act as strong oxidising agents.
(vii) Thermal Stability
The thermal stability of the interhalogen compounds depends upon the electronegativity difference between the two component halogen atoms. In general, the thermal stability of AX type interhalogens decreases as the electronegativity difference between them decreases.
For example: Thermal stability decreases as IF > BrF > CIF > ICl > IBr > BrCl
Structures of some common Interhalogen Compounds
(a) Neutral Interhalogens
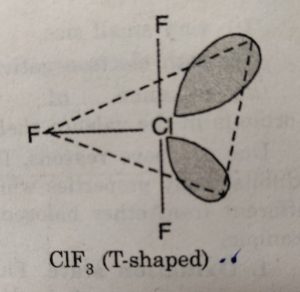
(i) XX3’ involves sp3d hybridisation of the central halogen atom and the molecule has trigonal bipyramidal geometry with two positions occupied by lone pairs. Its structure is termed as T-shaped.
For example: CIF3 has T-shaped structure.
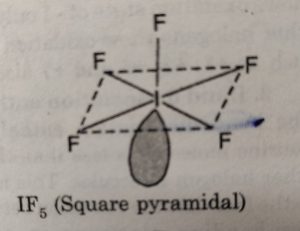
(ii) XX5‘ involves sp3d2 hybridisation of the central halogen atom and the molecule has octahedral geometry with one position occupied by a lone pair. Its structure is termed as square pyramidal.
For example: IF7 has square pyramidal structure.
(ii) XX7’ involves sp3d2 hybridisation of iodine atom and the molecule has pentagonal bipyramidal structure.
For example: IF7 has pentagonal bipyramidal structure.
(b) Interhalogen Ions
For example: ICl4¯ has square planar geometry.
Evidence of Cationic lodine
All halogens are non-metallic due to high electronegativities and ionisation energies. However, the last element iodine exhibits some properties showing the presence of positive ions. The positive iodine is found in unipositive (I+) and tripositive (I3+) states.
(i) Iodine monochloride (ICl) conducts electricity in the molten state. On electrolysis, iodine is liberated at the cathode while both iodine and chlorine are liberated at the anode. The liberation of Iodine at the cathode indicates the presence of cationic iodine. The ionisation of ICl may he represented as :
(ii) ICl3 conducts electricity and on electrolysis, iodine and chlorine are liberated at both the electrodes. Therefore, the jonization may be represented as
The ions ICl2+ and ICl4¯ contain I3+ ions.(iii) A large number of compounds containing iodine as I3+ have been isolated which are ionic.
Uses of Interhalogen Compounds
Pseudo Halogens
| Pseudo halogens | Pseudo halides |
| Cyanogen :(CN)2 | Cyanide : CN– |
| Thiocyanogen : (SCN)2 | Thiocyanate : SCN‾ |
| Oxycyanogen : (OCN)2 | Cyanate : OCN¯ |
Leave a Reply