Halides and Oxides of Phosphorus – The p-Block Elements – Class 12
Contents
Halides of Phosphorus
Phosphorus forms two types of halides i.e., phosphorus trihalides (covalency of P =3) PX3 (X = F, CI, Br, I) and phosphorus pentahalides PX5 (X = F, Cl, Br) (covalency of P=5).
With chlorine it forms:
(1) Phosphorus trichloride
(2) Phosphorus pentachloride.
Phosphorus Trichloride, PCl3
Preparation of Phosphorus Trichloride
(1) It is prepared in the laboratory by passing dry chlorine gas over heated white phosphorus. The vapours of PCl3 distil over and are collected in a receiver cooled by water.
P4 + 6Cl2 ——> 4PCl3
The receiver has a calcium chloride tube attached to it which protects it from the reactions of outside moisture. It can be purified by distilling over white phosphorus to remove the excess of chlorine.
(2) It can also be obtained by the reaction of thionyl chloride with white phosphorus.
P4 + 8SOCl2 —–> 4PCl3 + 4SO2 + 2S2Cl2
Thionyl chloride
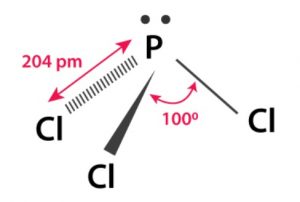
Structure of Phosphorus Trichloride
(1) Phosphorus in PCl3 undergoes sp3 hybridisation. Three of the sp3 hybrid orbitals overlap with p-orbitals of chlorine to form three P-Cl σ bonds while the fourth sp3 hybrid orbital contains a lone pair of electrons.
(2) PCl3 has pyramidal structure.
(3) The Cl P Cl bond angle in PCl3 is 100.4° which is greater than HPH bond angle in PH3 (93.6°). This is due to stearic crowding of two large Cl atoms in comparison to H atoms.
(4) The P-Cl bond is much larger (240 pm) than P-H bond (142 pm) because of larger size of CI atom.
Properties of Phosphorus Trichloride
Physical Properties
(i) It is a colourless oily liquid fuming constantly is the moist air.
(ii) Its specific gravity is 1.6.
(iii) Its boiling point and freezing point values are 347 K and 161 K respectively.
(iv) It has a highly pungent smell.
Chemical Properties of Phosphorus Trichloride
(1) Action with water
It fumes in moist air and reacts with water violently to form phosphorus acid.
PCl3 + 3H2O ——> H3PO3 + 3HCl
Phosphorous acid
(2) Action with atmospheric air or oxygen
It slowly combines with dry oxygen to form phosphorus oxychloride.
2PCl3 + O2 ——> 2POCl3
Phosphorous oxychloride
(3) Action with sulphur trioxide
It reacts with sulphur trioxide to form phosphorus oxychloride.
PCl3 + SO3 —–> POCl3 + SO2
Phosphorous oxychloride
(4) Action with chlorine or sulphur monochloride
It combines with chlorine or sulphur monochloride to form phosphorus pentachloride.
PCl3 + Cl2 —–>PCl5 + SO2
PCl3 + S2Cl2 —-> PCl5 + 2PSCl3
(5) Action with organic compounds
It reacts with organic compounds containing -OH group such as acetic acid, ethyl alcohol, etc.
(i) With organic acid, it forms acid chlorides.
3CH3COOH + PCl3 ——> 3CH3COCl + H3PO3
Acetic acid Acetyl chloride Phosphorus acid
(ii) With alcohols, it forms alkyl chlorides.
3C2H5OH + PCl3 ——> 3C2H5 + H3PO3
(6) It is readily oxidized to the important phosphorus (V) derivatives PCl5, POCl3 and PSCl3. It is oxidized by As2O3 to P2O5.
(7) PCl3 undergoes many substitution reactions and is main source of organophosphorus compounds.
Uses
It is mainly employed in the organic chemistry as an important reagent to replace the hydroxyl group (-OH) by chlorine atom in organic reactions.
Phosphors Pentachloride, PCl5
Preparation of Phosphors Pentachloride
(1) Phosphorus pentachloride is prepared by the reaction of white phosphorus with excess of dry chlorine.
P4 + 10Cl2 —–> 4 PCl5
(2) It can also be obtained by the reaction of dry chlorine on phosphorus trichloride.
PCl3 + Cl2 ——> PCl5
(3) Phosphorus pentachloride can also be prepared by the action of SO2Cl2 on phosphorus.
P4 + 10 SO2Cl2. ——–> 4PCl5 + 10SO2
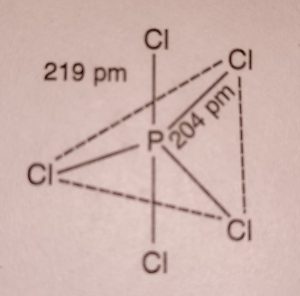
Structure Phosphors Pentachloride
(1) In PCl5, phosphorus undergoes sp3d hybridisation and has trigonal bipyramidal structure.
(2) It has three equatorial P-Cl bonds and two axial P-Cl bonds which are different.
Therefore, all the five P-Cl bonds are not equal. The axial bonds are larger than equatorial
bonds as :
P-Cl (axial) = 219 pm
P-Cl (equatorial) = 204 pm
(3) In gaseous and liquid phases, PCl5 has trigonal bipyramidal structure. However, in the solid state, it exists as an ionic solid [PCl4]+ [PCl6]¯. In this, the cation [PCl4]+ is tetrahedral and the anion [PCl6]¯ is octahedral.
(4) In solution, it exists either molecular or ionically dissociated depending on the nature of the solvent.
Physical Properties of Phosphorus Pentachloride, PCl5
(i) It is a yellowish white powder having pungent smell.
(ii) It fumes in moist air due to its strong affinity for water.
(iii) On heating, it sublimes at 433 K and can be melted (m.p. 318 K) only by heating it under pressure.
Chemical properties of Phosphorus Pentachloride, PCl5
(1) Stability
PCl5 is thermally less stable than PCl3. On heating it sublimes but decomposes on stronger heating into trichloride and chlorine.
PCl5 ⇔ PCl3
(2) Action with water
In moist air, it hydrolyses to POCl3 and finally gets converted to phosphoric acid.
PCl5 + H2O —-> POCl3 + 2HCl
Phosphorus oxychloride
POCl3 + 3 H2O —> H3PO4 + 3HCl
(3) Action with compounds containing OH group: It reacts with compounds containing OH group (-OH) to give the corresponding chloro compounds in which each-OH group is replaced by a chlorine atom.
For example
(i) With organic acid
CH3COOH +PCl5 ——> CH3COCl + POCl3 + HCl
(ii) with ethyl alcohol
C2H5OH + PCl5 —–> C2H5Cl + POCl3 + HCl
(iii) With sulphuric acid
OH-SO2-OH + 2PCl5 —-> ClSO2Cl + 2 POCl3 + 2HCl
(4) Reaction with phosphorus pentoxide and sulphur dioxide
PCl5 reacts with P4O10 forming phosphorus oxychloride and with SO2 forming phosphorus oxychloride and thionyl chloride.
6PCl5 + P4O10 ——> 10 POCl3
Phosphorus oxychloride
PCl5 + SO2 ——> POCl3 + SOCl2
(5) Reaction with phosphorus pentasulphide
PCl5 reacts with phosphorus pentasulphide to form phosphorus thiochloride.
6PCl5 + P4S10 ——> 10 PSCl3
(6) Reaction with metals
PCl5 reacts with finely divided metals on heating to give corresponding chlorides.
2Ag + PCl5 ——> 2 AgCl + PCl3
Sn + 2PCl5 —–> SnCl5 + 2PCl3
(7) Reduction
Phosphorus pentachloride is reduced with hydrogen to form PCl3
PCl5 + 2H ——-> PCl3 + 2HCl
(8) Reaction with potassium fluoride
With KF, PCl5 forms potassium phosphorus hexafluoride, K+[PF6]¯ + 5 KCl
Uses of Phosphorus Pentachloride
It is extremely useful in organic reactions to replace a hydroxyl group (-OH) by chlorine atom such as for the synthesis of C2H5Cl, CH3COCl etc.
Oxides of Phosphorus
Phosphorus forms two common oxides namely
(i) phosphorus trioxide (P4O6) and (ii) Phosphorus Pentaoxide (P4010)
(1) Phosphorus (III) oxide (P4O6)
Preparation of Phosphorus Trioxide
Phosphorus trioxide is formed when phosphorus is burnt in a limited supply of air.
P4 + 3O2 (limited) ———-> P4O6
Properties of Phosphorus Trioxide
(i) Phosphorus (III) oxide is a crystalline solid with garlic odour.
(ii) It is soluble in carbon disulphide, ether and chloroform.
(iii) Heating in air: On heating in air, it forms phosphorous pentaoxide.
P4O6 + 2 O2 —–> P4O10
(iv) Action of water: It dissolves in cold water to give phosphorous acid.
P4O6 + 6H2O (hot) —–>4H3PO4
It is, therefore, considered as anhydride of phosphorous acid. With hot water, it gives phosphoric acid and inflammable phosphine.
P4O6+ 6H2O (hot) ——> 3H3PO4 + PH3
(v) Action with Chlorine
It reacts vigorously with chlorine to form a mixture ‘of phosphoryl chloride and metaphosphoryl chloride.
P4O6 + 4Cl2 —->2POCl3 + 2PO2Cl
Phosphoryl chloride Metaphosphoryl chloride
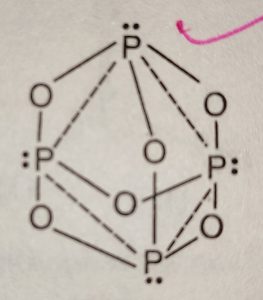
Structure of Phosphorus Trioxide
(1) Each atom of phosphorus in P4O6 is present at the corner of a tetrahedron.
(2) Each phosphorus atom is covalently bonded to three oxygen atoms and each oxygen atom is bonded to two phosphorus atoms.
(3) The six oxygen atoms lie along the edges of the tetrahedron of P atoms.
Phosphorus (V) Oxide (P4O10)
Preparation of Phosphorus Oxide
It is prepared by heating white phosphorus in excess of air.
P4 + 5O2 (excess) → P4O10
Properties of Phosphorus Oxide
(i) It is snowy white solid.
(ii) Action with water
It readily dissolves in cold water forming metaphosphoric acid
P4O10 + 2H2O (cold) —–> 4HPO3
Metaphosphoric acid
With hot water it gives phosphoric acid.
P4O10 + 6H2O (hot) —–> 4H3PO4
Phosphoric acid
(iii) Dehydrating Nature
Phosphorus pentaoxide has strong affinity for water and therefore, acts as a powerful dehydrating agent. It extracts water from many organic and inorganic compounds including sulphuric acid and nitric acid.
H2SO4 ——-> SO3
2HNO3 ——-> N2O5
2HClO4 —-> Cl2O7
CH3CONH2 ——-> CH3CN
Structure of Phosphorus Oxide
Its structure is similar to that of P4O10 . In addition, each phosphorus atom forms a double bond with oxygen atom.

Leave a Reply