Contents
Chlorine
Occurrence
Chlorine is very reactive and does not occur in nature in free state. It occurs mostly as chlorides of sodium and other alkali and alkaline earth metals. The most abundant compound of chlorine is sodium chloride (rock salt) and occurs in extensive evaporite deposits, saline lakes and brines and in the ocean. Other sources of chlorine are sylvine (KCl), carnallite (KCl.MgCl2.6H2O) etc.
Laboratory Preparation of Chlorine
Chlorine is rarely prepared in a laboratory on large scale because it is readily available in cylinders. However, when required it can be prepared by any one of the following methods :
(i) By heating manganese dioxide with concentrated hydrochloric acid
Chlorine is prepared easily by heating manganese dioxide with conc. HCl.
MnO2 + 4HCl —–>MnCl2 + Cl2 + 2H2O
A mixture of common salt and concentrated H2SO4 is used in place of HCl.
4NaCl+ MnO2+ H2SO4 —–>MnCl2 +4NaHSO4 + 2H2O + Cl2
Equal amounts of sodium chloride and manganese dioxide are finely ground and taken in a round bottomed flask. Concentrated sulphuric acid is poured from tap funnel and reaction mixture is heated gently. Greenish yellow vapours of chlorine gas rise up and are collected by the upward displacement of air.
(ii) By the action of HCl on potassium permanganate or potassium dichrormate.
2KMNO4 + 16HCl ——-> 2KCl + 2MNnCl2 + 8H2O + 5Cl2
K2Cr2O7+ 14HCl ——> 2KCl + 2CrCl3 + 7H2O + 3Cl2
(iii) By the action of HCl on lead oxides or bleaching powder.
PbO2 + 4HCl ——> PbCl2 +4H2O + Cl2
Pb3O4 + +8HCl ——->3PbCl2 +4H2O + Cl2
CaOCl2 + 2HCl —-> CaCl2 + H2O + Cl2
Manufacture of Chlorine
Chlorine is manufactured by the following methods:
(i) Deacon’s process: Hydrochloric acid is oxidized by atmospheric air in the presence of CuCl2 (catalyst) at 723 K.
4HCl + O2 ———->2Cl2 + 2H2O CuCl2 ,Catalyst 723 K
This method is modified by using improved catalyst (CuCl2 with didymium oxide as promoter)
(ii) Electrolytic process
Chlorine is obtained as a by-product during the manufacture of sodium hydroxide by the electrolysis of brine solution (sodium chloride solution).
NaCl ⇔ Na+ + Cl¯
H2O ⇔ H+ + OH¯
At cathode
H+ + e¯ ——> H
H + H ——-> H2
At anode
Cl‾ —-> Cl
Cl + Cl —-> Cl2
The sodium chloride solution contains Na+ and H+ (from water) yet the hydrogen ions are discharged more readily than Na+ ions at the cathode due to their low discharge potential.
Therefore, hydrogen gas is evolved at the cathode while solution becomes richer in OH¯ ions. Since Na+ ions were not discharged at the cathode, the concentration of Na+ ions also increase around the cathode.
Similarly, the Cl¯ ions are discharged more readily than OH¯ ions so that Cl2 gas evolved at the anode.
The Na+ and OH¯ ions in the solution combine to give sodium hydroxide.
Na+ + OH- —–> NaOH
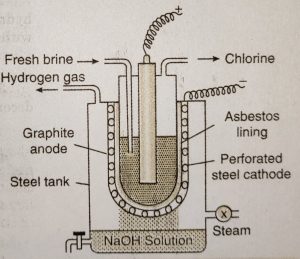
Since chlorine readily reacts with sodium hydroxide, it is necessary to devise some method to prevent the mixing of sodium hydroxide with chlorine. This is achieved by the use of porous diaphragm in a cell known as Nelson cell.
Nelson cell consists of a perforated U-shaped steel vessel which serves as a cathode.It is lined on the inside with porous diaphragm of asbestos. The cathode is suspended in a steel tank. Purified brine solution is taken in the steel cathode and a graphite anode is suspended in it.
When an electric current is passed through the brine solution, chloride ions (in NaCl) give electrons at the anode and change into chlorine. Sodium hydroxide formed around cathode seeps through the asbestos lining and fa!ls into the steel tank. Steam (which is being injected in the outer tank) helps in concentrating the solution of sodium hydroxide.
Properties of Chlorine
Physical properties of Chlorine
(i) It is greenish yellow gas.
(ii) It has strong pungent and suffocating odour.
(iii) It is a poisonous gas which causes headache.
(iv) It is about 2 times heavier than air.
(v) It is soluble in water and its aqueous solution is called chlorine water.It is also soluble in organic solvents like carbon tetrachloride. It can be liquefied easily into greenish yellow liquid which boils at 239 K.
(vi) Its b.p. is 239 K and m.p. is 171 K.
Chemical properties of chlorine
(1) Action on litmus : Dry chlorine has no effect on litmus paper. However, in the presence of moisture chlorine turns blue litmus red. This is due to fact that chlorine reacts with water to produce a mixture of hydrochloric acid and hypochlorous acid (HClO) which turns blue litmus red.
Cl2 + H2O —–> HCl + HClO
After turning blue litmus red, chlorine bleaches it because hypochlorous acid liberates nascent oxygen which converts the colouring matter into colourless matter.
HClO ——-> HCl + O
(2) Combination with hydrogen : Chlorine almost does not combine with hydrogen in the dark. However, in the presence of sunlight, chlorine combines with hydrogen explosively.
H2 + Cl2 ——> 2HCl
(3) Action with water : Since chlorine has great affinity for hydrogen, it decomposes water in presence of sunlight to produce hydrochloric acid.
2H2O + 2 Cl2 —> 4HCl + O2
(4) Combination with metals : Reactivity of chlorine towards different types of metals is as follows :
(i) Very active metals like sodium and calcium burn in the atmosphere of chlorine to form their chlorides.
2 Na + Cl2 → 2NaCl
Ca + Cl2 → CaCl2
(ii) Less active metals like iron, aluminum, zinc, copper or tin react with chlorine on heating.
2 Fe + 3 Cl2 —-> 2FeCl3
2Al + 3 Cl2 —> 2 AlCl3
Zn + Cl2 —-> ZnCl2
Sn+ Cl2 ——> SnCl2
Cu + Cl2 —> CuCl2
(5) Combination with non-metals : Non-metals like sulphur, phosphorus and boron react with chlorine readily.
S8 + 4 Cl2 ——-> 4S2Cl2
Sulphur monochloride
P4 + 10 Cl2 ——> 4PCl5
Phosphorus pentachloride
2B + 3Cl2 ———> 2 BCl3
(6) Combination with metalloids : Metalloids like arsenic and antimony catch fire in the atmosphere of chlorine to form their chlorides.
2As + 3Cl2 ——–> 2AsCl3
Antimony trichloride
2 Sb + 3Cl2 ——-> 2SbCl3
(7) Action with halides: Chlorine displaces the less electronegative halogens (bromine and iodine) from their metallic halides.
2KBr + Cl2 ——> 2KCl + Br2
2KCl + Cl2 —–> 2KCl + I2
(8) Action with alkalies :
(i) The reaction of chlorine with strong alkalies such as sodium hydroxide or potassium hydroxide depends upon the temperature of the reaction mixture.
(a) In cold, chlorine reacts with strong alkalies to form chlorides and hypochlorites as:
2NaOH + Cl2 ——-> NaCl + NaClO + H2O
Sod. hypochlorite
Similarly, the reaction of chlorine with cold solution of KOH is
2KOH + Cl2 —-> KCl + KClO + H2O
Pot. hypochlorite
(b) In hot, chlorine reacts with strong alkalies like NaOH or KOH to produce chlorides and chlorates as:
6NaOH + 3Cl2 ——> 5NaCl+ NaClO3 + 3H2O
Sod. chlorate
Similarly, the reaction of chlorine with hot KOH solution is
6KOH+ 3Cl2——–> 5KCl + KClO3 + 3 H2O
(ii) When chlorine gas is passed through hot milk of lime, calcium chloride and calcium chlorate are produced as :
6Ca(OH)2 + 6Cl2 ———> 5CaCl2 + Ca(ClO3)2 + 6H2O
Milk of lime Heat
However, if chlorine is passed through dry slaked lime, bleaching powder is produced.
2Ca(OH) + 2Cl2 —-> Ca(OCl)2 + CaCl2 + 2H2O
Slaked lime Bleaching powder
The composition of bleaching powder is
Ca(OC)2 , CaCl2. Ca(OH)2. 2H2O
(9) Action with ammonia: The reaction of chlorine with a ammonia depends upon the proportion of the reactants as
(i) If ammonia is in excess, nitrogen is formed
8NH8 + 3Cl2 ——> 6NH4Cl + N2
(excess)
(ii) If chlorine is in excess, nitrogen trichloride is formed.
NH3 + 3Cl2 ——-> NCl3 + 3HCl
(excess)
(10) As an oxidising agent: Since chlorine reacts with water and produces hydrochloric acid along with nascant oxygen, it acts as an oxidising agent.
Cl2 + H2O ——-> HCl + HClO
HClO——–> HCl + O
————————————
Cl2 + H2O —–> 2 HCl + O
————————————-
Some of its important oxidising reactions are:
(i) It oxidises sulphur dioxide to sulphuric acid.
Cl2 + SO2 + 2H2O ——-> 2HCl + H2SO4
(ii) It oxidises sulphites to sulphates.
Cl2 + H2O+ Na2SO3 ——> 2HCl + Na2SO4
(iii) It oxidises thiosulphates to sulphates.
Cl2 + H2O + Na2S2O3 ——> 2HCl + Na2SO4 + S
(iv) It oxidises hydrogen sulphide to sulphur.
Cl2 + H2S ——->2HCl + S
(v) It oxidises nitrites to nitrates.
Cl2 + H2O + NaNO2 ——-> 2HCl + NaNO3
(vi) It oxidises acidified ferrous salts to ferric salts.
2FesO4 + Cl2 + H2SO4 ——–> Fe2(SO4)3 + 2 HCl
(vii) It oxidises sodium arsenite (Na3AsO3) to sodium arsenate (Na3AsO4).
Na3AsO3 + Cl2 + H2O ——> Na3AsO4 + 2 HCl
(viii) It oxidises iodine to iodic acid.
I2 + 6H2O + 5Cl2 ——> 2HIO3 + 10 HCl
Chlorine can also act as an oxidising agent in absence of water.
(i) It oxidises ferrous chloride to ferric chloride.
2FeCl2 + Cl2 ——->FeCl3
(ii) It oxidises stannous chloride to stannic chloride.
SnCl2 + Cl2 ——–> SnCl4
(i) It oxidises potassium ferrocyanide to potassium ferricyanide.
2K4[Fe(CN)6] + Cl2 ——->2 K3[Fe(CN)6] + 2KCl
(iv) It oxidises potassium manganate to potassium permanganate.
2K2MnO4 + Cl2 → 2KMNO4 + 2KCl
(11) Addition reactions : Chlorine reacts with many substances like carbon monoxide, nitric oxide and sulphur dioxide in presence of sunlight to form addition products:
CO + Cl2 ———> COCl2
Carbonyl chloride (phosgene)
2NO + Cl2 ——-> 2NOCl
Nitrosyl chloride
SO2 + Cl2 ———-> SO2Cl2
Sulphuryl chloride
(12) Reaction with other halogens : Chlorine can reacts with other halogens like F, Br, or I, to form interhalogen compounds.
For example,
Cl2 + F2 —> 2 ClF
Cl2 + 3F2 —> 2ClF3
Br2 + Cl2 –> 2 BrCl
I2 + Cl2 ——> 2ICl
(13) Action with organic compounds : Reaction of chlorine with organic compounds depends upon the nature of the organic compound.
(i) With saturated compounds like methane, chlorine produces substituted products.
CH4+Cl2 ——-> CH3Cl + HCl
(ii) With unsaturated compounds like ethene, ethyne, chlorine forms addition products :
C2H4 + Cl2 ——> C2H4Cl2
ethene 1,2-Dichloroethane
C2H2 + 2Cl2 ——–> C2H2Cl4
ethyne 1,1,2,2-tetrachloroethane
(iii) Organic compounds like turpentine oil (C10H16) when introduced in the atmosphere of chlorine gas catches fire.
C10H16 + 8Cl2 —-> 16 HCl + 10C
(14) Bleaching agent: Chlorine bleaches natural colouring matter of green leaves, flowers, indigo, litmus in presence of water. Its bleaching action is due its ability to oxidise the colouring matter to colourless matter as:
Cl2 + H2O —->2HCl + O
Colouring matter + O —–> Colourless matter
—————————————————————–
Colouring matter +Cl2 + H2O –> Colourless matter + 2HCl
—————————————————————–
Some noteworthy points regarding its bleaching action are :
(i) Chlorine bleaches only in the presence of water. Thus, dry chlorine cannot bleach dry flowers because in absence of water, it cannot produce nascent oxygen needed for oxidation.
(i) Since chlorine bleaches by oxidation, the bleaching action of chlorine is permanent.
(iii) Since during bleaching by chlorine, hydrochloric acid is produced, delicate articles are likely to be destroyed by hydrochloric acid. Therefore, chlorine is used only for bleaching paper pulp, wood pulp and cloth.
Uses of Chlorine
(i) Large quantities of chlorine are used industrially for bleaching wood pulp (required for manufacture of paper and rayon), bleaching of cotton, paper, wood, textiles, etc.
(ii) It is used for the manufacture of dyes, drugs, refrigerants, etc.
(iii) In the manufacture of chlorinated organic solvents such as chloroform (CHCl3), carbon tetrachloride (CCl4), etc. which are used for dry cleaning and degreasing machinery.
(iv) In the manufacture of chlorates which are used in flash light powders matches and explosives.
(v) In the manufacture of bleaching powder, aluminium chloride and sodium hypochlorite which are important industrial compounds.
(vi) In the manufacture of vinyl chloride which is a starting material for polyvinyl chloride plastics.
(vii) In sterilisation of drinking water.
(viii) In the manufacture of D.D.T. which is an important insecticide.
(ix) In the manufacture of poisonous gases like phosgene (COCl2), tear gas (CCl3.NO2) and mustard gas (ClCH2CH2SCH2CH2Cl)
(x) In the extraction of metals like platinum and gold.
Leave a Reply