Contents
Chemical Reactivity
The halogens react readily with metals and non-metals to form halides. Fluorine is the most reactive of all the halogens. The reactivity of the halogens decreases down the group.
The high reactivity of halogens is due to the following reasons :
(i) Low dissociation enthalpies
All the halogens have very low dissociation enthalpies. As a result, they can readily dissociate into atoms and react with other substances.
(ii) High electron gain enthalpy
Halogens have very high negative electron gain enthalpy values and therefore, have very strong tendency to gain an electron. Thus, halogens are very reactive elements due to their low dissociation enthalpies and high negative electron gain enthalpies. Fluorine has the lowest bond dissociation enthalpy. This is due to weak F-F bond because of the repulsion between the non-bonding electrons in the small molecule. Therefore, it is most reactive among the halogens. Halogens have high electron acceptance property and therefore they have strong tendency to take up the electron :
½ X2 + e¯ ——> X¯
As a result, they act as powerful oxidising agents. Fluorine is the strongest oxidising agent and oxidises other halide ions in solution or even in the solid phase. In general, a halogen of lower atomic number will oxidize halide ion of higher atomic number and therefore, will liberate them from their salt solutions as given below :
F2 + 2X¯ —> 2 F¯ + X2 (X = Cl, Br, I)
Cl2 + 2X¯ —> 2 Cl¯ + X2
Br2 + 2 I¯ ——> 2 Br¯ + I2
The decreasing oxidising power of the halogen as we go down the group is shown by their decreasing reduction potentials.
F2 + 2e¯ —> 2 F¯ E° = + 2.87 V
Cl2 + 2e¯ —> 2 Cl E° = +1.36 V
Br2 + 2e‾ —-> 2 Br¯ E° = + 1.09 V
I2 + 2e‾ —-> 2 I¯ E° = + 0.54 V
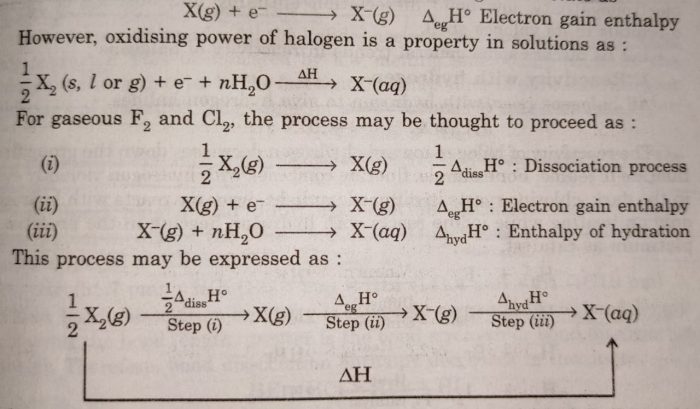
The electrode potential of F2 is maximum while that of I2 is the minimum.This means that F2 can be reduced most easily and I2 is reduced least readily. This means that F2 is the strongest oxidising agent while I2 is the weakest oxidising agent. Chlorine has the highest negative electron gain enthalpy, so gaseous Cl atoms have maximum tendency to accept electrons and therefore, chlorine is expected to be strongest oxidising agent. However, chlorine is not strongest oxidising agent, but fluorine is the strongest oxidising agent. This can be explained with the help of Born Haber cycle.
The energy is needed to dissociate or convert molecular halogen into atomic halogen. The enthalpy change for this step is positive. Flourine has less negative electron gain enthalpy, yet it is strongest oxidising agent because of the following reasons :
(i) F2 has low enthalpy of dissociation because of weak F—F bond.
(ii) F2 has very high enthalpy of hydration because of smaller size of the F¯ ion.
The larger amount of energy released in step (iii) and lesser amount of energy required in step (i) overweighs the smaller energy released in step (ii) for fluorine. As a result, AH overall is more negative for fluorine than for chlorine. Thus, fluorine is very strong oxidising agent. The values of enthalpy changes of different steps are given below :
Since bromine occurs as liquid at room temperature, it involves enthalpy of fusion also, while iodine which is solid at room temperature, involves enthalpy of sublimation as well as enthalpy of fusion. The overall energy released for these are less than those of F2 and Cl2.
The relative oxidising power of halogens can be further illustrated by their reactions with water. Fluorine is so strong oxidising agent that it oxidises water to dioxygen. The reaction is spontaneous and strongly exothermic.
2F2(g) + 6H2O(l) ——-> 4H3O+ (aq) + 4F¯ (aq) + O2(g)
The reaction with chlorine and bromine are thermodynamically possible but they react very slowly forming corresponding hydrohalic and hypohalous acids.
X2(g) + H2O(l) ——–> HOX(aq) + HX(aq) (X = Cl or Br)
The reaction of iodine with water is non-spontaneous. In fact, iodide can be oxidised by oxygen in acidic medium.
4I¯ (aq) + O2(g) + 4H+(aq) —-> 2 I2 (s) + 2 H2O (l)
Anomalous behaviour of fluorine is attributed to its small size, highest electronegativity, low bond dissociation enthalpy and absence of vacant d-orbitals in its valence shell.
(1) Reactivity with Hydrogen
All halogens react with hydrogen to give hydrogen halides.
H2 + X2 —–> 2HX
The reactivity of halogen towards hydrogen decreases down the group from fluorine to iodine. For example: fluorine combines with hydrogen violently even in the dark, chlorine reacts in diffused sunlight, bromine reacts with hydrogen only on heating while iodine reacts with hydrogen heating in the presence of platinum as catalyst.
H2 + F2 ——> 2HF
H2 + Cl2 ——> 2HCl
H2 + Br2 —-> 2HBr
H2 + I2 —–>2HI
Hydrogen fluoride and hydrogen chloride are manufactured by heating a mixture of calcium fluoride (fluorite or fluorospar) and sodium chloride with concentrated H2SO4 respectively.
CaF2 (s) + H2SO4 (aq) —-> CaSO4 (s) + 2HF (g)
2NaCl (s) + H2SO4 (aq) → Na2SO4 (s) + 2HCl (g)
HBr is manufactured by direct reaction of HI and Br2 at about 570 K in the presence of platinum catalyst.
H2(g) + Br2(g)———-> 2HBr
HI is manufactured by the reaction of HI with H2S or hydrazine.
2I2 (s) + N2H4 (aq) —–> HI (aq) + N2 (g)
Properties of hydrogen halides
(i) Physical state
Hydrogen fluoride is a low boiling liquid (b.p. 292 K) while HCl, HBr and HI are gases. The anomalous property of HF is due to presence of hydrogen bonding in the molecules. Due to hydrogen bonding in HF molecules it exists as associated molecule (HF)n.
(ii) Melting and boiling points
The boiling point of HF is highest (b.p. 293 K) due to extensive intermolecular hydrogen bonding. The other halides show negligible hydrogen bonding because of the lower electronegativity of the halogen atom in them. Therefore, the boiling points of HCl, HBr and HI are much lower than that of HF. As we move from HCl to HI, the boiling points regularly increase down the group. They are held together by weak van der Waals’ forces which increase with increasing size of halogen atom.
The melting point of HF is higher than that of HCl. The melting points of other halides increase gradually from HCl to HI as the size of halogen atom increases.
(iii) Nature of bonds
All the halides are covalent compounds with some ionic character. The degree of ionic character decreases in the order:
HF > HCl > HBr > HI
(iv) Bond length and bond dissociation enthalpy
As the size of halogen atom increases, the bond length (H-X) increases in the same order.
The bond length of HX molecule increases as :
HF (91.7 pm) < HCl (127.4 pm) < HBr (141.4 pm) < HI (160.9 pm)
Now, bond dissociation enthalpy is inversely proportional to bond length i.e., shorter the bond length, greater is the bond strength or bond dissociation enthalpy. Therefore, bond dissociation enthalpy decreases in the order:
HF > HCl > HBr > HI
(v) Thermal stability
The thermal stability of the hydrides decreases from HF to HI. HF is most stable whereas HI is least stable. For example, HF and HCl are stable up to 1500 K while HBr dissociates to the extent of 10% and HI is dissociated to the extent of 20% at 700 K.
The decrease in stability of the hydrides is due to decrease in bond strength which decreases when we go down the group.
(vi) Reducing character
The decreasing thermal stability of hydrogen halides from HF to HI indicates that the reducing character increases down the group as
HF < HCI < HBr < HI
Thus, HF is not a reducing agent at all. HCl is a weak reducing agent, HBr is a stronger reducing agent while HI is the strongest reducing agent among all the hydrides.
(vii) Acidic strength
In gaseous state, hydrogen halides are covalent. But in aqueous solutions, they ionise and behave as acids. The acidic strength of these acids decreases in the order :
HI > HBr > HCl > HF
Thus, HF is the weakest acid and HI is the strongest acid among these hydrogen halides.
Explanation: Fluorine is the most electronegative halogen, therefore, the electronegativity difference will be maximum in HF and should decrease gradually as we move towards iodine through chlorine and bromine. Thus, HF should be more ionic in nature and consequently it should be strongest acid. The bond dissociation energy decreases from HF to HI so that HF has maximum bond dissociation energy and HI has the lowest value.
Since H-I bond is weakest, it can be easily dissociated into H+ and I– ions while HF can be dissociated with maximum difficulty. Thus, HI is the strongest acid while HF is the weakest acid among the hydrogen halides.
(2) Reactivity towards Oxygen
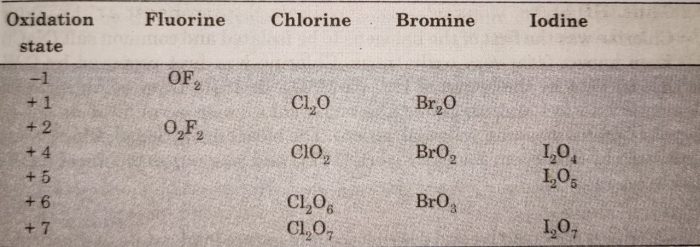
Halogens form many binary compounds with oxygen but most of these are unstable. Fluorine forms two oxides OF2 and O2F2. However, only OF2 is thermally stable at 298 K while O2F2 is highly unstable and decomposes into elements even at 113 K. These oxides are called oxygen fluorides because fluorine is more electronegative than oxygen. Both these fluorides are strong fluorinating agents. O2F2 oxidises plutonium to PuF6 and this reaction is used in removing Pu as PuF6 from spent nuclear fuel.
H2S + 4 O2F2 —-> SF6 + 2 HF + O2
On the other hand, the oxides of chlorine, bromine and iodine are called oxides. They form oxides from +1 to +7 oxidation states.
Chlorine forms the largest number of oxides while iodine forms the least oxides. In these binary compounds, the bonds are mainly covalent because of small difference in electronegativity between the halogens and oxygen. However, the bond polarity increases as we move from chlorine to iodine because of increasing difference in electronegativity.
The stability of oxides formed by halogens decreases as :
I > Cl > Br
Bromine oxides are least stable. Iodine-oxygen bond is stable because of greater polarizability of I while the stability of chlorine oxygen bond is due to multiple bond formation involving d-orbitals of Cl atom.
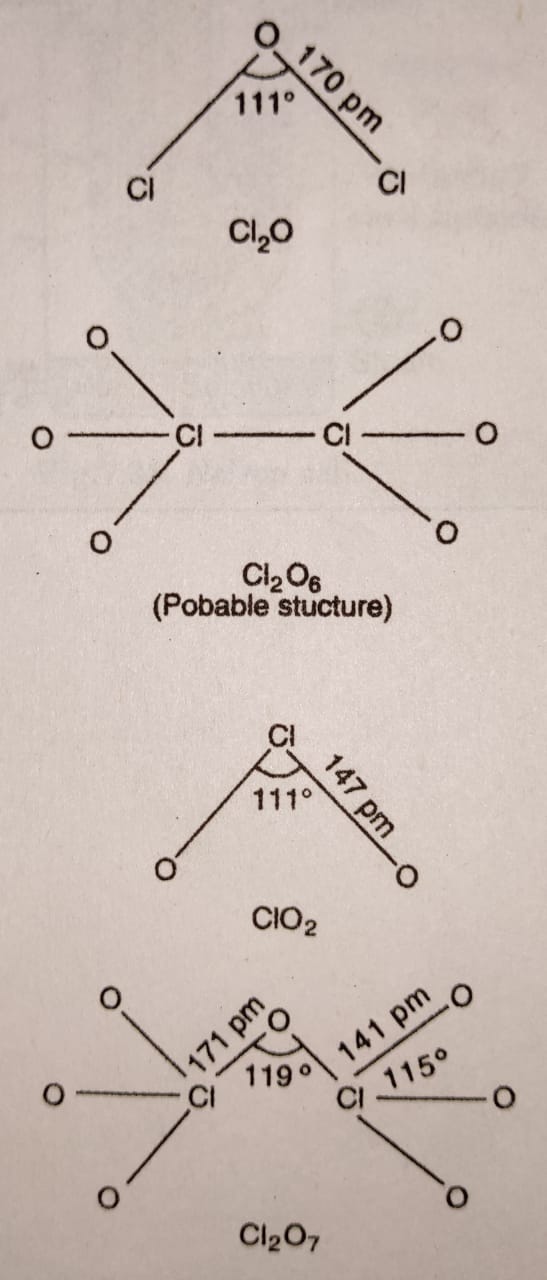
Chlorine dioxide is the only one prepared on a large scale by the reduction of ClO3¯ with SO2 in strongly acidic medium :
2NaClO3 (aq) + SO2 (g) ——> 2ClO2 (g) + Na2SO4(aq)
All these oxides of chlorine are powerful oxidising agents and decompose explosively when subjected to mechanical shock or heat. ClO2 and Cl2O are used as bleaching agents for paper, pulp, textiles and water treatment.
The oxides of bromine Br2O, BrO2, BrO3 etc. are the least stable halogen oxides and exist only at lower temperatures. The oxides of iodine, I2O4, I2O5 and I2O7 are insoluble and decompose on heating. For example: I2O5 decomposes on heating above 673 K
I2O5 —————-> 2 I2 + 5 O2
It is strong oxidising agent. It oxidises H2S to sulphur and HCl to chlorine.It oxidises CO to CO2 quantitatively liberating iodine which can be titrated against sodium thiosulphate.
I2O5 +5CO ——–> I2 + 5CO2
(3) Reactivity towards Metals
Halogens react with metals to form metal halides. For example: Bromine reacts with magnesium to give magnesium bromide.
Mg(s) + Br2 (l) → MgBr2(s)
Fluorine is most reactive and the reactivity decreases as we move down the group.The ionic character of the metal halides decreases in the order :
MF > MCI > MBr > MI
If a metal exhibits more than one oxidation states, the halides in higher oxidation state will be more covalent than the one in the lower oxidation state. For example: SnCl4, PbCl4 , SbF4, and UF6, are more covalent than SnCl2, PbCl2 ,SbCl3 and UF6.
(4) Reactivity of Halogens towards Halogens
Halogens combine amongst themselves to form a number of compounds known as interhalogen compounds. These interhalogen compounds are of the type AX, AX3, AX5 and AX7 where X is large size halogen atom showing negative oxidation state.
Leave a Reply