Chemical Properties of Group 15 Elements – The p-Block Elements – Class 12
Contents
Chemical Properties of Group 15 Elements
Nitrogen differs from rest of members of the group due to its
(1) smaller size,
(2) high electronegativity,
(3) high ionisation enthalpy and
(iv) non-availability of d-orbitals in the valence shell.
Nitrogen has unique tendency to form pπ-pπ multiple bonds with itself and with other elements having small size and high electronegativity (e.g., C and O).
However, the heavier elements of this group do not form pπ-pπ bonds because their atomic orbitals are so large and diffuse that they cannot have effective overlapping.
Anomalous Properties of Nitrogen
Nitrogen is a colourless gas and exists as diatomic. The two nitrogen atoms are held together by triple bond (one σ and two π) between two atoms and have very high bond enthalpy. Due to the presence of triple bond, which has very high bond enthalpy, the nitrogen molecule is inert and has very low reactivity. Phosphorus, arsenic and antimony exist in various forms containing single bonds as P-P, As-As and Sb-Sb.
For example: Phosphorus exists as P4 tetrahedral molecules. In this case, four P atoms lie at the corners of a regular tetrahedron. Each P is bonded to three P atoms by single P-P bonds. However, the single N-N bond is weaker than the single P-P bond because of high inter electronic repulsions of non bonding electrons owing to small bond length (109 pm). As a result, the catenation tendency is weaker in nitrogen. Therefore, nitrogen exists as gas white phosphorus exists as solid.
Since P-P single bond is much weaker than NΞN triple bond, therefore, phosphorus is much more reactive than nitrogen.
White phosphorus is more reactive than nitrogen. It catches fire when exposed to air, burning to form the oxide, P4O10. It is stored under water to prevent it. Red P is stable in air at room temperature but reacts on heating.
Arsenic and antimony both occur in two forms. The most reactive is yellow form which contains M4, tetrahedral units and resembles white phosphorus. Arsenic is stable in dry air but tarnishes in moist air giving first a bronze and then a black coating on its surface.
Antimony is leas reactive and is stable towards water and air at room temperature. On heating in air, it forms Sb406, Sb408 or Sb4O10.
Nitrogen does not have d-orbitals in its valence shell. Nitrogen cannot form pπ-dπ bonds whereas Phosphorus and other heavier members of this group readily form pπ-dπ multiple bonds.
For example: Phosphorus forms compounds containing pπ-dπ bonds such as R3P=O, R3P=CH2 (R=alkyl group), POX3 (X = F, CI, Br), etc. Phosphorus and arsenic can also form dπ-dπ bonds with transition metals where their compounds like P(C2H5)3 and As(C6H5)3 act as ligands.
1. Reactivity towards Hydrogen (formation of hydrides)
All the elements of group 15 form gaseous trihydrides of the formula EH3, (where E=N, P, As, Sb or Bi) such as:
NH3 Ammonia
AsH3 Arsine
SbH3 Stibine
PH3 Phosphine
BiH3 Bismuthine
The lighter elements also form hydrides of the formula M2H4 such as N2H4 (hydrazine), P2H4 (diphosphine) and As2H4 (diarsine).
Nitrogen also forms a special hydride of the formula HN3, known as hydrogen azide or hydrazoic acid.
Preparation
The trihydrides can be easily obtained by the hydrolysis of their binary metal compounds with water or dilute acids:
MgN2 + 6H20 ——-> 3Mg(OH)2 + 2NH3
Ca3N2 +6H20 ——–> 3Ca(OH)2 + 2NH3
Ca3P2 +6H20 ——–> 3Ca(OH)2 + 2PH3
Zn3E2 + 6 HCl —–> 3 ZnCl2
The trichlorides of these elements except that of bismuth give the corresponding hydrides on reduction with Zn/acid or LiAlH4.
ECl3, +3LiAlH4 ——> EH3 + 3LiCl+ 3AlH3 (E=N,P, As, Sb)
Ammonia (NH3) is the most important trihydride of the group and is extensively used in the manufacture of nitric acid (HNO3) and important chemical fertilizers such as ammonium sulphate, urea, calcium ammonium nitrate, calcium cyanamide, etc.
It is prepared on an industrial scale by Haber process. In this process, nitrogen combines with hydrogen at 650-800 K under a pressure of 200-350 atm in the presence of iron catalyst and Mo as promoter.
N2 + 3H2 ——-> 2NH3
Δ H =-92.4 KJ/mol
Conditions: 650-800 K, 200-350 Atm, Fe catalyst , Mo
Phosphine (PH3) can be prepared by heating white phosphorus with concentrated caustic alkali in an inert atmosphere of oil gas:
P4+3KOH +3H2O —–> PH3 +3KH2PO2
Structure
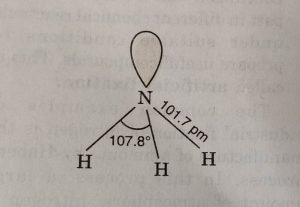
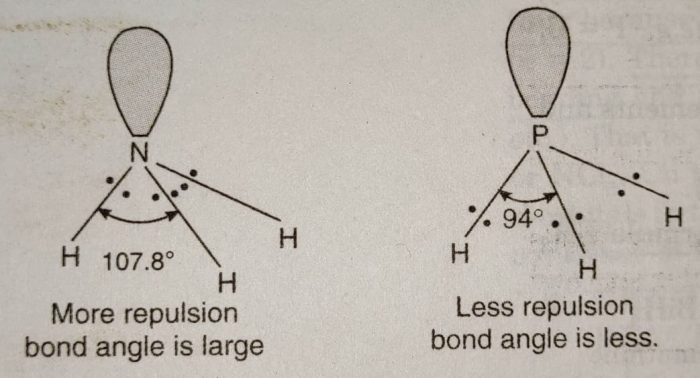
All these hydrides are covalent in nature and have pyramidal structure. These involve sp³ hybridization of the central atom and one of the tetrahedral position is occupied by a lone pair. Due to the presence of lone pair, the bond angle in NH3, is less than the normal tetrahedral angle. It has been found to be 107°. As we go down the group the bond angle decreases as:
NH3 PH3 AsH3 SbH3 BiH3
107.8° 93.6° 91.8° 91.3° 90°
Explanation: In all these hydrides, the central atom is surrounded by four electron pairs, three bond pairs and one lone pair. Now, as we move down the group from N to Bi, the size of the atom goes on increasing and its electronegativity decreases. The position of bond pair shifts more and more away from the central atom in moving from NH3, to BiH3.
For example: The bond pair in NH3, is close to N in N-H bond than the bond pair in P-H bond in PH3. As a result, the force of repulsion between the bonded pair of electrons in
NH, is more than in PH. In general, the force of repulsion between bonded pairs of electrons decreases as we move from NH3 to BiH3 and therefore, the bond angle also decreases in the same order.
Characteristics of Hydrides
The important characteristics of these hydrides are :
1) Basic strength: All these hydrides have one lone pair of electrons on their central atom. Therefore, they act as Lewis bases. They can donate an electron pair to electron deficient species (Lewis acids). As we go down the group, the basic character of these hydrides decreases.
For example: NH3 is distinctly basic; PH3 is weakly basic AsH3, SbH3, and BiH3, are very
weakly basic.
Explanation: Nitrogen atom has the smallest size among the hydrides. Therefore, the lone pair is concentrated on a small region and electron density on it is the maximum. Consequently, its electron releasing tendency is maximum.
As the size of the central atom increases down the family, the electron density also decreases. As a result, the electron donor capacity or the basic strength decreases down the group.
(ii) Thermal stability: Thermal stability of the hydrides of group 15 elements decreases as we go down the group. Therefore, NH3 is most stable and BiH3 is least stable.
The stability of the hydrides of group 15 elements decreases in the order:
NH3 > PH3 > AsH3 > SbH3 > BiH3
Explanation: On going down the group, the size of the central atom increases and therefore, its tendency to form stable covalent bond with small hydrogen atom decreases. As a result the M-H bond strength decreases and therefore thermal stability decreases.
(iii) Reducing character: The reducing character of the hydrides of group 15 elements increases from NH3 to BiH3. Thus, increasing order of reducing character is as follows :
NH3 < PH3 < AsH3 < SbH3 < BiH3
Explanation: The reducing character depends upon the stability of the hydride. The greater instability of a hydride, the greater is its reducing character. Since the stability of group 15 hydrides decreases from NH3 to BiH3 hence the reducing character increases.
For example: NH3 being most stable among the group 15 hydrides is not a good reducing agent. Ammonia at high temperatures reduces copper oxide to copper :
3CuO + 2NH3 ——> 3Cu + N2 + 3H20
(iv) Boiling and melting points: Ammonia has a higher boiling point than phosphine and then the boiling point increases down the group because of increase in size.
Explanation: The abnormally high boiling point of ammonia is due to its tendency to form hydrogen bonds.
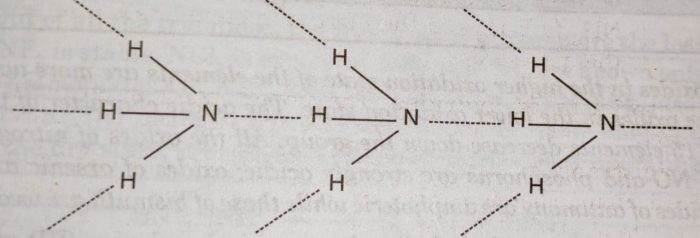
In PH3 and other hydrides, the intermolecular forces are van der Waals forces. These vander Waal’s forces increase with increase in molecular size and therefore, boiling points increase on moving from PH3 to BiH3.
(v) Solubility: Ammonia forms hydrogen bonding with water molecules while phosphine and other hydrides do not form hydrogen bonding with water.
NH3 is soluble in water while PH3 and other hydrides are insoluble in water.
2. Reactivity towards Oxygen (formation of oxides)
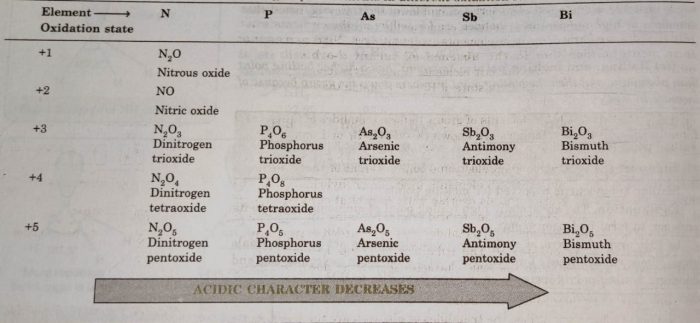
The elements of group 15 combine with oxygen directly or indirectly to form two types of oxides, E2O3 (trioxides) and E2O5 (pentaoxides).
Nitrogen forms a number of oxides with oxidation states ranging from +1 to +5 because of strong tendency of nitrogen to form pπ-pπ multiple bonds with oxygen.
The oxides in the higher oxidation state of the elements are more acidic than that of the oxides in the lower oxidation state. The acidic character of the oxides of group 15 elements decrease down the group. All the oxides of nitrogen. except N2O and NO and phosphorus are strongly acidic; oxides of arsenic are weakly acidic; oxides of antimony are amphoteric while those of bismuth are weakly basic.
The two common oxides of phosphorus are phosphorus (III) oxide and phosphorus (V) oxide, Phosphorus trioxide, P4O6 is a dimer of P2O3 and is prepared by heating white phosphorus in limited supply of oxygen.
P4 + 3O2 ——-> P4O6
Phosphorus (V) oxide is a dimer of P2O5 and is prepared by heating white phosphorus in excess of air or oxygen.
P4+ 5O2 (excess) —–> P4O10
Both P4O10 and P4O6 are acidic oxides which dissolve in water to give phosphonic acid (or phosphorous acid) and phosphoric acid (ortho phosphoric acid) respectively.
P4O6 + 6H2O —–> 4H3PO4
P4010+ 6H20 ———> 4H3PO3
Because of strong affinity for water, P4O10 is used as a dehydrating agent. It can dehydrate HNO3 and H2SO4 to give N2O5 and SO3 respectively.
As4O6 and Sb4O6 are obtained by burning the metals in air or oxygen.Both are very poisonous. The stability of higher oxidation state decreases on descending the group.
The basic nature of oxides increases with increasing atomic number.
For example: P (III) and As (III) oxides are acidic, Sb (III) oxide S amphoteric and Bi (III) oxide is distinctly basic. It dissolves in acids to form salts.
Bi2O3+ 6HNO3 —–>2Bi(NO3)3 + 3H2O
3. Reactivity towards Halogens (formation of halides)
Group 15 elements form two series of halides of the type MX3 (trihalides) and MX5 (pentahalides)
Nitrogen cannot form pentahalides due to the absence of vacant d-orbitals in its outermost shell. Bi has little tendency to form pentahalides because + 5 oxidation state of Bi is less stable than +3 oxidation state due to inert pair effect.
(a) Trihalides: All the elements of group 15 form trihalides of the general formula EX3.All these trihalides are known (X = F, CI, Br or I and E = N, P, As, Sb, Bi).
Structure
1) The valence shell electronic configuration of these elements is ns2 npx1 npy1 npz1 and all these elements undergo sp3 hybridisation.
2) The three of the four sp3 hybrid orbitals overlap with 2p orbital of halogen atom to form three σ bonds.
3) The fourth sp3 hybrid orbital contains the lone pair of electrons. Therefore, the geometry of trihalides may be regarded as pyramidal.
Properties of Trihalides
(i) The trihalides of group 15 elements are predominantly covalent with the ionic character increasing down the group.
For example: BiF3 is ionic while other halides of Bi, i.e., BiCl3, BiBr3, etc. and SbF5 are partly covalent and partly ionic.
(ii) The trihalides of nitrogen are the least stable.Though NF3 is stable, NCl3 is explosive. NBr3, and NI3, are known only as their unstable ammoniates i.e., NBr3.6NH3 and NI3.6NH3
(iii) The nitrogen triiodide ammoniate is stable only in the moist state. In the dry state, it explodes with noise when struck liberating vapours of iodine.
3NI3.6NH3 ——> N2 + 3I2 + 12NH3
NCl3, NBr3 and NI3 are unstable because N-X bond is weak due to large difference in the size of N and X atoms.
NF3 is stable because of small difference in size of N (75 pm) and F (72 pm) resulting strong N-F bond. Because of stability of NF, it behaves quite differently from others.
It is unreactive and does not hydrolyse with water, dilute acids or alkalies. PF3 is rather less reactive towards water and is more easily handled than the other halides.
(iii) The trihalides are easily hydrolysed by water. However, the products are different in hydrolysis of different chlorides.
NCl3 + 3H2O —–> NH3 + 3 HClO
PCl3 + 3H2O —–> H3PO3 + 3HCl
AsCl3 + 3 H2O —> As2O3 + 6 HCl
Antimony and bismuth trichlorides are only partially hydrolysed to form oxychlorides.
SbCl3 + H2O ⇔ SbOCl + 2 HCl‾
BiCl3 + H2O ⇔ BiOCl + 2 HCl
(iv) The trihalides of P, As and Sb (especially fluorides and chlorides) act as Lewis acids and combine with Lewis bases :
PF3 + F2 —> PF5
SbF3 + 2F¯ ——> [SbF5]¯
SbCl3 + 2 Cl¯ —–> [SbCl5]2¯
(b) Pentahalides:
P, As and Sb form pentahalides of the general formula EX5, EF5 (E = P, As, Sb) ECl5 (E =P, As and Sb) and PBr3 and PI3. N does not form pentahalides because of the absence of d-orbitals in its valence shell.
The pentahalides of group 15 elements are thermally less stable than trihalides. This is due to the following reasons:
(i) As we go down the group, the stability of +5 oxidation state decreases while that of +3 oxidation state increases due to inert pair effect. Therefore, the pentahalides of Bi are not known except for BiF5.
(ii) As the size of halogen atom increases from F to I, the strength of E-X bond decreases and the stearic hindrance increases. As a result pentabromides and pentaiodides are unstable.
Pentafluorides of P, As, Sb and Bi are known, while bromides and iodides of only P are known.
Preparation
The pentahalides are prepared as:
3PCl3+ 5 AsF3 ——-> 3PF3 + 5AsCl3
PCl3 + Cl2 —> PCl5
2As2O3 + 10F2 —–> 4ASF5 + 3O2
2Sb2O3 + 10 F2 —–> 4 SbF5 + 3 O2
2Bi + 5F2 —-> 4BiF2
Structure
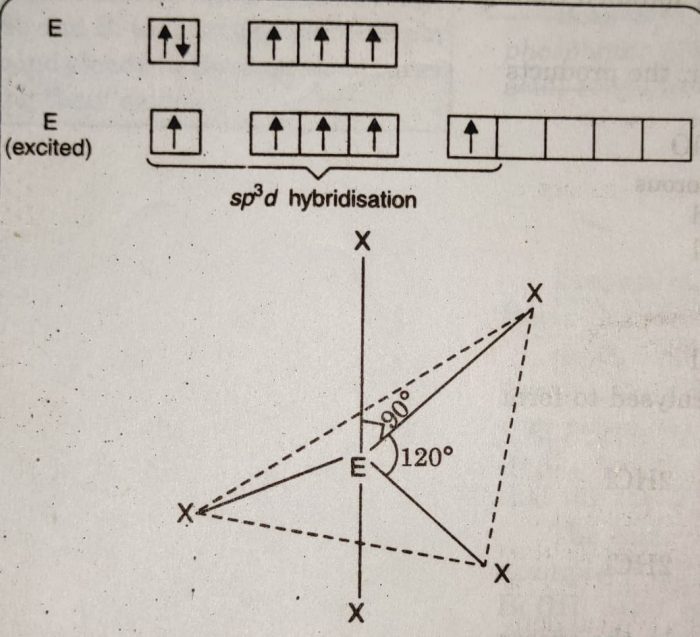
1) The pentahalides of group 15 elements have trigonal bipyramidal geometry in which the central atom is sp3d hybridised.
2) The three halogen atoms occupy equatorial positions while the other two occupy axial positions.
3) The axial bonds are at 90° while equatorial bonds are at 120° each. The axial positions experience greater repulsion than equatorial positions by bond pair of electrons.
4) As a result, axial bonds are usually larger than the equatorial bonds. In which P-F axial bond lengths are 158 pm while P-F equatorial bond lengths are 153 pm.
Properties
1) Pentahalides are thermally less stable than their corresponding trihalides. PF5 is molecular in both the gaseous and solid states. PCl5 exists as molecules in the gas phase but exists as [PCl4]+[PCl6]¯ in the crystalline state.
2) PBr5 and PI5 also exist in the ionic form as [PBr4]+ [PBr6]¯ and [PI4]+I¯ respectively in the solid state.
3) All the pentahalides behave as Lewis acids because of the presence of vacant d-orbitals. The central atom (except N) can accept a pair of electrons thereby expanding its coordination number to 6.
EX5 + X¯ ——–> [EX6]¯
During this the hybridisation of the central atom changes from sp3d to sp3d2
4. Reactivity towards Metals
All the elements of group 15 combine with metals to form their binary compounds in which the elements show -3 oxidation state.
For example: Nitrogen forms nitrides (Mg3N2 : magnesium nitride, Ca3P2 : calcium nitride)
arsenic forms arsenides (Na3As: sodium arsenide), antimony forms antimonides (Zn2Sb3) , zinc antimonide and bismuth forms bismuthides (Mg3Bi2: magnesium bismuthides).
Leave a Reply