Contents
Representative Elements or the Main Group Elements
In the long form of the periodic table, the elements have been classified into four blocks : s, p, d and f depending upon the subshell in which the last electron enters.
The elements belonging to s and p-blocks in the periodic table are called representative elements or the main group elements. They belong to groups 1, 2 and from 13 to 18.
The elements belonging to groups 1 and 2 belong to s- block and have the general configuration ns1-2. The elements belonging to groups 13 to 18 belong to p-block and have the general configuration ns2 np1-6.
Group 15 Elements
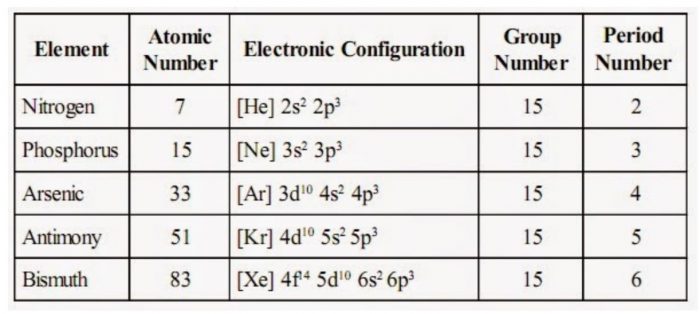
It contains five elements namely nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb) and bismuth (Bi). This group is regarded as nitrogen family.
Occurrence
Molecular nitrogen comprises about 78% by volume of the Earth’s atmosphere, it is not very abundant in the earth’s crust.Since nitrates are very soluble in water so these are not widespread in the earth’s crust. The only major nitrate minerals are NaNO3 and KNO3
Nitrogen is also an important constituent of proteins, amino acids and nucleic acids in plants and animals. The continuous interchange of nitrogen between the atmosphere and biosphere is called nitrogen cycle.
Phosphorus is the eleventh element in order of abundance in crystal rocks of the earth. All its known minerals are orthophosphates.
The common minerals of phosphorus are:
(i) Phosphorite Ca3(PO4)2
(ii) Fluorapatite Ca5(PO4)3F or Ca3(PO4)2CaF2
(iii) Chlorapatite Ca5(PO4)3Cl or 3Ca3(PO4)2. CaCl2
(iv) Hydroxyapatite : Ca5(PO4)3OH or 3Ca3(PO4)2.Ca(OH)2
Phosphorus is essential for life, both as a structural material in animals and plants. It is present in bones as well as in living cells. About 60% bones and teeth are Ca3(PO4)2 or [3{Ca3(PO4}2.CaF2].
It also occurs in nucleic acids (DNA and RNA) which control the hereditary effects in human beings. Phosphorus is also found in ATP (adenosine triphosphate and ADP (adenosine diphosphate).
The elements arsenic, antimony and bismuth are not very abundant. These are obtained as metallurgical by-products from roasting sulphide ores.
General characteristics of Group 15 elements
Electronic Configurations
The atoms of group 15 have five electrons in the outermost shell, two in s and three in p subshell.
The general electronic configuration of this group may be expressed as ns2 np3.
Atomic and Physical Properties
(1) Atomic and ionic radii : The atomic and ionic radii of group 15 elements are smaller than the atomic radii of the corresponding group 14 elements.
On going down the group, the atomic radii increase with increase in atomic number.
Explanation: The nuclear charge in case of elements of group 15 is larger than in the case of elements of group 14. Due to increased nuclear charge, the electrons are strongly attracted by the nucleus and therefore, atomic radii decrease.Thus, the atomic radii of elements of group 15 are less than those of group 14.
On moving down the group, the atomic radii increase due to increase in number of shells because of addition of a new principal shell in each succeeding element.
However, from As to Bi only a small increase in covalent radius is observed. This is due to the presence of completely filled d and or f-orbitals in the heavier members.
(2) lonisation enthalpies: The first ionisation enthalpies of the group 15 elements are higher than the corresponding members of the group 14 elements.
On going down the group, the ionisation enthalpies decrease.
Explanation: The larger ionisation enthalpy is due to greater nuclear charge, small size and stable configuration of the atoms of group 15 elements.
The electronic configuration of atoms of group 15 are half filled, npx1, npy2 npz1 and are stable. Therefore, they have high ionisation enthalpies.
The decrease in ionisation enthalpy, as we move down the group, is due to increase in atomic size and screening effect which overweigh the effect of increased nuclear charge.
(3) Electronegativity: The electronegativity values of elements of group 15 are higher than the corresponding elements of group 14.
On going down the group, the electronegativity value decreases.
Explanation: The elements of group 15 have smaller size and greater nuclear charge of atoms and therefore, they have higher electronegativity values.
The decrease in electronegativity on going down the group is due to increase in size of the atoms and shielding effect of inner electron shells on going down the group.
(4) Metallic character: The elements of group 15 are less metallic. However, on going down the group, the metallic character increases from N to Bi. For example: N and P are non-metallic, As and Sb are partly non-metallic while Bi is a metal.
Explanation: Due to increased nuclear charge and higher electronegativity, the elements of group 15 are less metallic than the corresponding elements of group 14.
On moving down the group, the atomic size as well as the screening effect of the intervening electrons increases. As a result, the ionization enthalpy decreases and therefore, metallic character increases down the group.
(5) Melting and boiling points: The melting points of group 15 elements first increase from nitrogen to arsenic and then decreases to antimony and bismuth. However, the boiling points increase regularly on going from nitrogen to bismuth.
Explanation: The melting points increase down the group due to increase in atomic size However, the unexpected decrease in the melting points of Sb and Bi is because of their tendency to form three covalent bonds instead of five covalent bonds, due to inert pair effect. As a result, the attraction among their atoms is weak and hence their melting points are low. Because of large size of atoms, Bi has still weaker interatomic forces than Sb and therefore, has still lower melting point.The boiling points increase down the group from N to Bi because of increase in their atomic size.
(6) Catenation: The elements of group 15 also show a tendency to form bonds with itself (self linking of atoms) known as catenation. For example: hydrazine (H2NNH2) has two N atoms bonded together, hydrazoic acid (N3H) has three N-atoms, azide ion, N3¯ has also three N atoms bonded together
Among the elements of group 15, phosphorus has the maximum tendency for catenation forming cyclic as well as open chain compounds consisting of many phosphorus atoms.
The lesser tendency of elements of group 15 to show catenation in comparison to carbon is their low (M-M) bond dissociation enthalpies.
(7) Allotropy: Except nitrogen and bismuth, all other elements of this group show allotropy.
For example:
a) phosphorus exists as white, black or red phosphorus.
b) arsenic exists as yellow or grey arsenic
c) antimony exists as yellow or silvery grey allotropic forms.
Oxidation States
The elements of group 15 have five electrons in their valence shell.They exhibit various oxidation states from -3 to +5.
(i) Negative oxidation states
These elements have five electrons in the valence shell (ns2np3) and therefore, require three more electrons to acquire the nearest noble gas configuration. But, the gain of three electrons is not energetically favourable because it requires very large amount of energy to gain three electrons and form M3- ions.
(1) Nitrogen being the smallest and most electronegative element of the group forms N3–(nitride) ion and shows an oxidation state of 3 in nitrides of some highly electropositive metals such as Mg3N2, Ca3N2, etc.
(2) The other elements of this group form covalent compounds even with metals and show an oxidation state of -3 with metals. For example: calcium phosphide (Ca3P2), sodium arsenide (Na3As), zinc antimonide (Zn3Sb2), magnesium bismuthide (Mg3Bi2).
(3) The tendency of the elements to exhibit -3 oxidation state decreases on moving down from P to Bi due to increase in size and metallic character. The last member of the group, Bi hardly forms any compound in -3 oxidation state.
(4) In addition to -3 oxidation state, N and P show oxidation states of -2 in hydrazine (NH2NH2) and diphosphine (P2H4) respectively.
Nitrogen also shows an oxidation state of -1 in hydroxyl amine (NH2OH) but P does not.
(ii) Positive oxidation states
(1) All the elements of group 15 exhibit positive oxidation states of +3 and +5. However, on moving down the group, the stability of +5 oxidation state decreases while that of +3 oxidation state increases due to inert pair effect.
Because of energy consideration, these elements cannot lose all the five valence electrons. Therefore, they do not form M5+ ions and all the compounds of group 15 elements having +5 oxidation state (i.e., PF5, PCl5, SbF5, BiF5) are essentially covalent compounds.
(2) Nitrogen does not form compounds in +5 oxidation state such as NF5, NCl5, etc. because it does not have vacant d-orbitals in its valence shell which can enable it to extend its octet. The stability of the highest oxidation state (+5) decreases down the group. The +5 oxidation state of Bi is less stable than in Sb. This is due to inert pair effect.
(3) The elements of group 15 form both covalent (e.g., NCl3, PCl3, AsCl3, SbCl3) and ionic compounds (e.g., BiF3, SbF3) in +3 oxidation state. The +3 oxidation state becomes more and more stable on moving down the group.
(4) Nitrogen and phosphorus also show oxidation state of +4 because of the ability of one lone pair on NH3 and PH3 to form dative bonds with Lewis acids. However, nitrogen can exist in various oxidation states from -3 to +5 in its hydrides, oxides and oxo acids.
(5) Phosphorus also exhibits +1 and +4 oxidation states in some oxo acids. In nitrogen, all oxidation states from +1 to +4 tend to disproportionate in acid solution. In P nearly all the intermediate oxidation states disproportionate into +5 and -3 both in acid and alkali solutions.
(6) The maximum covalency of nitrogen is restricted to four because it does not have vacant d-orbitals in its outermost valence shell (n = 2). Therefore, only four (one 2s and three 2p) orbitals are available for bonding and it cannot extend its valency beyond four. That is the reason why nitrogen does not form pentahalides such as NF5 or NCl5. On the other hand, phosphorus and all other elements have vacant d-orbitals in their valence shells and can use all their valence orbitals to exhibit covalency of five or six e.g., PF5, PCl5, AsF5, PF6¯, [SbF6]‾, etc.
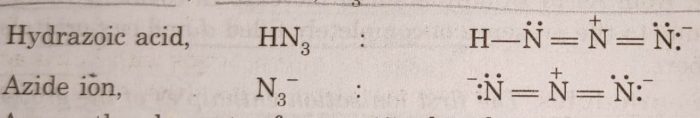
Very Helpful!
very nice, thanks from all of us.
It was very helpful for my project
Indeed this website is helpful and it concisely explains in a clear and organized pattern.Thank you.
Very impressive it has really assisted me