Contents
Allotropes of Sulphur
|
(i) Rhombic sulphur
(iii) Plastic sulphur
(ii) Monoclinic sulphur
|
(i) Rhombic sulphur or α-Sulphur
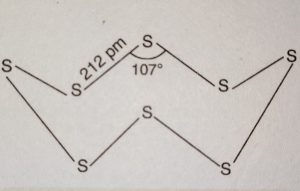
(ii) Monoclinic sulphur or β-Sulphur
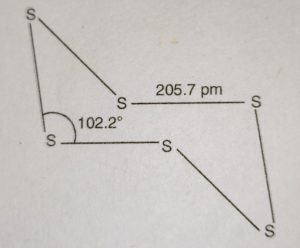
(iii) Plastic sulphur or δ-sulphur
Sulphur Dioxide
Laboratory Preparation of Sulphur Dioxide
Cu + 2 H2SO4 → CuSO4 + SO2 + H2O
Physical Properties of Sulphur Dioxide
Chemical Properties of Sulphur Dioxide
Sod. sulphite
The gas is non-combustible and does not support combustion. However, certain substances such as carbon, magnesium, etc. extract oxygen from the gas and burn in it when ignited.
Uses of Sulphur Dioxide
(1) It is used in the manufacture of important chemicals such as sulphuric acid , sodium hydrogen sulphite, calcium hydrogen sulphite etc. These bisulphites are used for preservatives for jams, pickles, jellies.
(2) It is used for refining of petroleum and sugar.
(3) It is used for bleaching delicate articles such as wool, silk , straw etc.
(4) It is used as a disinfectant and germicides.
(5) It is as an antichlor i.e. Foe removing excess chlorine from bleached articles.
(6) Liquid SO2 is used as a solvent to dissolve a number of organic and inorganic chemicals.
(7) Liquid SO2 is used as a refrigerant because it can be liquified and re-evaporated easily.
This is the best work, thank you
This has really helpful.Thank you