Contents
Potassium Dichromate
Properties of Potassium Dichromate
1) Colour and melting point : It is an orange red crystalline solid having melting point of 670 K.
2) Solubility : It is moderately soluble in cold water but readily soluble in hot water.
3) Action of heat : It decomposes on heating to form potassium chromate , chromic acid and oxide.
4 K2Cr2O7 → 4K2CrO4 + 2 Cr2O3 + 3 O2
4) Action of alkalies : On heating with alkalies, the orange colour of dichromate solution changes to yellow due to the formation of chromate ion.
4 K2Cr22O7 + 2KOH → 2 K2CrO4 + H2O
Cr2O72- + 2 OH¯ → 2 CrO42- + H2O
On acidifying the yellow solution , the colour again changes to orange red due to the reversible reaction.
2 K2CrO4 + H2SO4 → K2Cr2O7 + K2SO4 + H2O
2 CrO42- + 2 H+ → Cr2O72- + H2O
5) Chromyl chloride test
When potassium dichromate is heated with conc. sulphuric acid and a soluble metal chloride , orange red vapours of chromyl chloride are evolved.
4 K2Cr22O7 + 4 NaCl + 6 H2SO4 → 2 KHSO4 + 4 NaHSO4 + 2 CrO2Cl2 + 3 H2O
6) Action with Hydrochloric acid
Potassium dichromate reacts with hydrochloric acid and evolve chlorine
K2Cr22O7 + 14 HCl → 2KCl + 2CrCl3 + 7H2O + 3 Cl2
7) Reaction with hydrogen peroxide
Acidified potassium dichromate reacts with hydrogen peroxide to give a deep blue solution due to the formation of [CrO(O2)2] or CrO5
Cr2O72- + 4 H2O2 + 2 H+ -→ 2CrO5 + 5 H2O
The blue colour fades away gradually due to the decomposition of CrO5 to Cr3+ and oxygen.
8) Oxidising character
The dichromates act as a powerful oxidising agents in acidic medium. In the presence of dilute sulphuric acid , K2Cr2O7 liberates nascent oxygen and therefore acts as an oxidising agent.
K2Cr22O7 + H2SO4 → K2SO4 + Cr2(SO4)3 + 4H2O + 3(O)
The Cr2O72- ion takes up electron in the acidic medium and hence acts as an oxidising agent.
Cr2O7 2- + 14H+ + 6e- → 2Cr3+ + 7 H2O
Cr2O7 2- ( Cr in +6 oxidation state) is itself reduced to Cr3+ (Cr in +3 oxidation state) and its equivalent weight will be :
Equivalent weight of K2Cr22O7 = Molecular weight / 6 = 294 /6 = 49
K2Cr22O7 is used as a primary standard in volumetric analysis because it is hygroscopic.
Oxidising reaction of K2Cr22O7
1) It liberates I2 from KI
K2Cr2O7 + H2SO4 → K2SO4 + Cr2(SO4)3 + 4H2O + 3O
2KI + H2SO4 + O → K2SO4 + H2O + I2 ] × 3
____________________________
6KI + K2Cr2O7 + 7H2SO4 → 4 K2SO4 +Cr2(SO4)3 + 7H2O + 3 I2
____________________________
Cr2O7 2- + 14 H+ + 6e– → 2Cr3+ + 7 H2O
6I– → 3I2 + 6 e–
____________________________
Cr2O7 2- + 14 H+ + 6I– → 2Cr3+ + 7 H2O + 3I2
____________________________
2) It oxidises ferrous salts to ferric salts
K2Cr2O7 + H2SO4 → K2SO4 + Cr2(SO4)3 + 4H2O + 3O
2FeSO4 + H2SO4 + O → Fe2(SO4)3 + H2O ] × 3
____________________________
6 2FeSO4 + K2Cr2O7 + 7 H2SO4 → 3 Fe2(SO4)3 + K2SO4 + Cr2(SO4)3 + 7 H2O
____________________________
or ferrous to ferric ions
6 Fe2+ + Cr2O7 2- + 14 H+ —–> 6 Fe3+ + 2 Cr3+ + 7 H2O
3) It oxidises hydrogen sulphide to sulphur
K2Cr2O7 + 4 H2SO4 → K2SO4 + Cr2(SO4)3 + 4H2O + 3O
H2S + O → H2O + S ] × 3
____________________________
3 H2S + K2Cr2O7 + 4 H2SO4 → K2SO4 + Cr2(SO4)3 + 3 S + 7 H2O
____________________________
3 H2S + Cr2O7 2- + 8 H + → 2 Cr3+ + 3 S + 7 H2O
4) It oxidises sulphites to sulphates
K2Cr2O7 + 4 H2SO4 → K2SO4 + Cr2(SO4)3 + 4H2O + 3O
Na2SO3 + O -→ Na2SO4 ] × 3
____________________________
3 Na2SO3 + K2Cr2O7 + 4 H2SO4 → 3 Na2SO3 + K2SO4 + Cr2(SO4)3 + 4H2O
____________________________
3 SO32- + Cr2O7 2- + 8 H+ → 2 Cr3+ + 3 SO42- + 4H2O
It also axidises thiosulphates to sulphates and sulphur
3 S2O32- + Cr2O7 2- + 8 H+ → 2 Cr3+ + 3 SO42- + 4H2O + 3S
It oxidises , chlorides to chlorine , nitrites to nitrates , sulphur dioxide to sulphuric acid , thiosulphates to sulphates and sulphur.
Cr2O7 2- + 6Cl¯ + 14H+ → 2 Cr3+ + 3 Cl2 + 7 H2O
Cr2O7 2- + 3 NO2¯ +8 H+ → 2 Cr3+ + 3 NO3¯ + 4 H2O
Cr2O7 2- + 3 SO2 + 2H+ → Cr2(SO4)3 + H2O
5) It oxidises stannous salt to Stannic salts
Cr2O7 2- + 14H++ 3 Sn2+ → 2 Cr3+ + 3 Sn4+ + 7 H2O
6) It oxidises ethyl alcohol to acetaldehyde and acetic acid
K2Cr2O7 + 4 H2SO4 → K2SO4 + Cr2(SO4)3 + 4H2O + 3 O
CH3CH2OH + O → CH3CHO + H2O
CH3CHO + O → CH3COOH
Structure of Chromate and Dichromate Ions
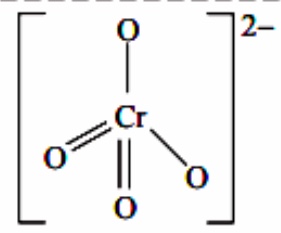
1) The chromate ion CrO42- has tetrahedral structure in which four atoms around Cr atoms are oriented in a tetrahedral arrangement. All the four Cr–O bonds are equivalent.
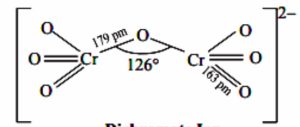
2) The dichromate ion consist of two tetrahedral units sharing one corner with Cr-O-Cr bond angle of 126°
3) These two Cr-O bonds are larger than the other six equivalent Cr-O bonds.
Structure of Chromate ion
Structure of Dichromate ion
Uses of Potassium Dichromate
1) It is used in dyeing and calico printing
2) It is used in chrome tanning in leather industry.
3) For the preparation of a large number of chromium compounds such as chrome yellow , chrome red , chrome alum , zinc yellow etc.
4) It is used as a volumetric reagent in laboratory in redox titration for the estimation of ferrous ions , iodide ions.
5) In photography for hardening gelatin film.
6) A mixture of K2Cr2O7 and conc H2SO4 is used as a cleaning mixture in the laboratories.
7) In organic chemistry as an oxidising agent.
Your notes are really helpful ☺️☺️☺️☺️☺️☺️☺️
They very related to NCERT.
YOUR NOTES ARE PERFECT .