Contents
Chemical Reactivity
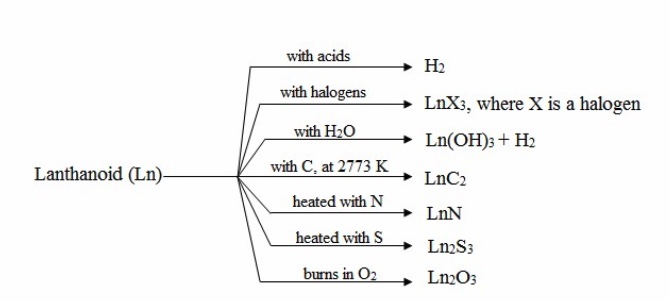
Chemical characteristics of Lanthanoids
1) All the lanthanoids are silvery white soft metals which tarnish readily on exposure to air. In finely divided state, all burn in air to form sesquioxides of the formula Ln2O3 (where Ln is lanthanoid) except cerium which gives CeO2 , Ytterbium, however, resists the action of air even at 1275 K due to the formation of a protective coating of its oxide.2) The hardness of lanthanoids increases with increasing atomic number. Samarium is very hard like steel. Their melting point range between 1000-1275 K but samarium melts at 1623 K.
3) They have typical metallic structure and are good conductors of heat and electricity.
4) In their chemical behaviour, the earlier members of the series are quite reactive similar to calcium but with increasing atomic number they behave like aluminium. The e.m.f. values for the half reaction are in the range of – 2.2 to – 2.4 V except for Ln = Eu for which the value is -2.0 V.
Ln 3+(aq) +3e → Ln (s)
5) All these metals combine with hydrogen when heated gently in the gas (473- 573 K) to form LnH3.6) They combine with halogens and sulphur to form LnX3, Ln2S3 .
7) All react with water slowly in cold but rapidly on heating liberating hydrogen and forming Ln(OH)3.
8) They combine with nitrogen to form nitrides of the formula, LnN.
9) When the metals are heated with carbon, they form carbides of the formula Ln3C, Ln2C3, and LnC2.
10) They liberate hydrogen from dilute acids.
Solubility of Compounds
1) The fluorides, oxides, hydroxides, carbonates, phosphates, chromates and oxalates of lanthanoids are largely insoluble in water.2) The halides other than fluorides, nitrates, acetates, perchlorates and salts of oxoacids of lanthanoids follow the pattern of solubility of salts of group 2 elements.
3) However, lanthanoids sulphates unlike the sulphates of group 2 elements are soluble in water.
Basic Character of Hydroxides
All the lanthanoids form hydroxides of formula Ln(OH)3. These are ionic and basic in character. They are stronger bases than Al(OH)3, but weaker than Ca(OH)3
Since the ionic size decreases from La3+ to Lu3+ , the basicity of hydroxides decreases in the same order. Thus, La(OH)3 is the strongest base while Lu(OH)3, is the weakest base.
Leave a Reply