Contents
Trends in Stability of Higher Oxidation States
1) In metal halides
The transition elements react with halogens at high temperatures to form transition metal halides. These reactions have very high activation energies, therefore, higher temperatures are required to start the reaction. But once the reaction starts, the heat of reaction is sufficient to continue the reaction.
The halogens react in the order:
Order of reactivity: F2 > Cl2 > Br2 > I2
| Element | Oxidation Number | Compounds |
| Sc | +6 | ScX3 |
| Ti | +4 , +3 , +2 | TiX4 , TiX3 , TiX2 |
| V | +2 , +3 , +4 , +5 | VX2 , VX3 , VX4 , VX5 |
| Cr | +2 , +3 , +4 , +5 ,+6 | CrF6 , CrF5 , CrX4 , CrX3 , CrX2 |
| Mn | +2 , +3 , +4 | MnX2 , MnF3 , MnF4 |
| Fe | +3 , +2 | FeX3 , FeX2 |
| co | +2 , +3 | CoF3 , CoX2 |
| Ni | +2 | NiX2 |
| Cu | +1 , +2 | CuX2 , CuX |
| Zn | +2 | ZnX2 |
The second and third transition series elements exhibit higher coordination numbers and their higher Oxidation states are more stable than corresponding first transition series elements.
a) The elements of first transition series trend to exist in low oxidation states. Chromium to zinc form stable difluorides and the other chlorides are also known.
b) Since fluorine is the most electronegative element, the transition metals show highest oxidation states with fluorine. For example : CrF6 and VF5
c) The highest oxidation states are found in TiX4 (tetrahalides, X =F , cl , Br and I) , VF5 and CrF6
d) The +7 oxidation state for Mn is not shown by simple halides. However MnO3F is known in which the oxidation state of Mn is +7.
e) After Mn, the tendency to show higher oxidation states with halogens are uncommon. Iron and cobalt form trihalides FeX3 (X = F, Cl or Br) and CoF3
f) The tendency of fluorine to stablise the highest oxidation state is due to either higher lattice enthalpy as in case of CoF3 or higher bond enthalpy due to higher covalent bonds e.g. VF5 and CrF6
g) V(V) is shown by VF5 only. However, the other halides undergo hydrolysis to form oxohalides, VOX3
h) Fluorides are relatively unstable in their low oxidation states.
For example: Vanadium forms only VX2 (X = Cl, Br or I ) and copper can form CuX (X = CI, I). All copper (II) halides are known except the iodide. This is because, Cu2+ oxidises I– to I2.
Many copper (I) compounds are unstable in aqueous solution and they undergo disproportionation to Cu(ll) and Cu (0) as:
Copper in +2 oxidation state is more stable than in +1 oxidation stateThis can be explained on the basis of much larger negative hydration enthalpy (ΔhydHφ) of Cu2+ (aq) than Cu+, which is much more than compensates for the large energy required to remove the second electron i.e. So ionisation enthalpy of copper.
2) In metal oxides and oxo cations
The ability of oxygen to stabilize the highest oxidation state is remarkably exhibited in their oxides. The highest oxidation states in their oxides coincides with the group number.
For example : The highest oxidation state of scandium of group 3 is +3 in its oxide , Sc2O3
The highest oxidation state of manganese of group 7 is +7, in Mn2O7. However beyond group 7, no higher oxides of iron above Fe2O3 are known.
Though higher oxidation state such as +6 is shown in ferrates such as Fe3O42- in akaline medium, but they readily decompose to Fe2O3 and O2. Oxocations of the metals also stabilise higher oxidation states.
For example: V(IV) as VO2+ , V(VI) as V and T(III) as TiO2+. The ability of oxygen to stabilise these high oxidation states exceeds that of fluorine.
For example : Manganese forms highest fluoride as MnF4, whereas the highest oxide is Mn2O7. This is due to the tendency of oxygen to form multiple bonds. In the covalent oxide, Mn2O7, each Mn is tetrahedrally surrounded by oxygen atoms and has Mn-O-Mn bridge. The tetrahedral [MO4]n- ions are also known for vanadium, (V) chromium (VI), manganese (VI) and manganese (VII).
Magnetic Properties
The magnetic properties of a compound are a measure of the number of unpaired electrons in it. When a magnetic field is applied, there are two main types of substances:
(a) Paramagnetic substances: The substances which are attracted by magnetic field are called paramagnetic substances and this character arises due to the presence of unpaired electrons in the atomic orbitals.
(b) Diamagnetic substances : The substances which are repelled by magnetic filed are called diamagnetic substances and this character arises due to the presence of paired electrons in the atomic orbitals.
Most of the compounds of transition elements are paramagnetic n nature and are attracted by the magnetic field.
Explanation for paramagnetic character of transition elements
The transition elements involve the partial filling of d-subshells. Most of the transition metal ions or their compounds have unpaired electrons in d-subshell (from configuration d1 to d9) and therefore, they give rise to paramagnetic character.
The magnetic character is expressed in terms of magnetic moment. The larger the number of unpaired electrons in a substance, the greater is the paramagnetic character and larger is the magnetic moment. The magnetic moment is expressed in Bohr magnetons abbreviated as B.M.
Paramagnetism arises from the presence of unpaired electrons. Each unpaired electron have magnetic moment associated with its spin angular momentum and orbital angular momentum. Therefore, for the first transition series elements, the magnetic moments arise only from the spin of the electrons.
This can be calculated from
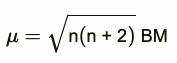
Leave a Reply