Contents
Werner’s Coordination Theory
The earlier studies of cobalt complexes were precipitation reactions, conductance measurements and isomeric behaviour.
(1) Precipitation Studies
The number of ions furnished by a complex in a solution can be determined by precipitation reactions.
For example:
(b) When the compound CoCl3.6NH3 is treated with excess of AgNO3 , 3 mol of AgCl are obtained from 1 mol of the compound i.e. all the three Cl¯ ions are precipitated.
(c) When the compound CoCl3.5NH3 is treated with excess of AgNO3, 2 mol of AgCl are obtained i.e., only two Cl¯ ions are precipitated. This means that the compound CoCl3.5NH3 has three ionizable chloride ions whereas in the compound CoCl3.5NH3 only two chlorine atoms are ionizable as Cl¯ ions.
Similarly, the number of chloride ions precipitated in the case of the compounds CoCl3.4NH3 and CoCl3.3NH3 have been found to be 1 and none.
(2) Conductance measurements
For example: The complex CoCl3.6NH3 behaved as 1:3 electrolyte, CoCl3.5NH3 as 1:2 electrolyte, CoCl3.4NH3 as 1:1 electrolyte.
(3) Isomers of compounds
Postulates of Werner’s Coordination Theory
(2) Every metal atom has a fixed number of secondary valencies i.e., it he fixed coordination number.
(3) The metal atom tends to satisfy both its primary as well as secondary valencies. Primary valencies are satisfied by negative ions whereas secondary valencies are satisfied either by negative ions or by neutral molecules. In certain cases, a negative ion may satisfy both types of valencies.
(4) The secondary valencies are always directed towards the fixed position in space and this leads to definite geometry of the coordination compound. Secondary valencies have characteristic spatial arrangements corresponding to different coordination numbers. Spatial arrangements are called coordination polyhedra.
For example: If a metal ion has six secondary valencies, these are arranged octahedrally around the central metal ion. If the metal ion has four secondary valencies, these are arranged in either tetrahedral or square planar arrangement around the central metal ion. The secondary valencies, thus, determine the stereochemistry of the complex.
Thus, a metal atom exhibits primary valencies in the formation of its salts (e.g., CoCl3 , AgNO3) while the metal atom exhibits its secondary valencies in the formation of its complex ions
Structures of Coordination Compounds on the Basis of Werner’s Theory
![structure of [Co(NH3)6]Cl3](https://classnotes.org.in/wp-content/uploads/CoNH36Cl3-.jpg)
The primary valencies are ionizable and therefore, all the chloride ions would get precipitated on the addition of silver nitrate.
The species within the square brackets are also called coordination entities (or complexes). The ions outside the square brackets are called counter ions. Thus, in the coordination compound [Co(NH3)6Cl]3, [Co(NH3)6]3+ represents coordination entity and 3Cl¯ ions represent counter ions
The ionisation of the coordination compound is written as:
(2) CoCl3.5NH3 : In this compound, the coordination number of cobalt is 6 but now five positions are occupied by NH3 molecules and the sixth position by one of the chloride ions.This chloride ion has dual character as it satisfies secondary as well as a primary valency as indicated by a full line as well as a dotted line.The two Cl¯ ions satisfy the remaining two primary valencies of cobalt. This satisfies 6 secondary and 3 primary valencies of cobalt.However, on ionisation, only two Cl¯ ions will be precipitated because one Cl- ion which also satisfied secondary valency, will not be precipitated.
![structure of [Co(NH3)6]Cl3](https://classnotes.org.in/wp-content/uploads/CoCl3.5NH3.jpg)
(3) CoCl3.4NH3 : In the compound CoCl3.4NH3 , two chloride ions exhibit dual character of satisfying both primary and secondary valencies. It will give precipitate with silver nitrate corresponding to only one Cl‾ ion and the number of ions in this case is 2.
![structure of [CoCl2(NH3)4]Cl2](https://classnotes.org.in/wp-content/uploads/CoCl2NH34Cl2.jpg)
It may be formulated as
(4) CoCl3.NH3 : In the compound CoCl3.3NH3 ,three chloride ions satisfy primary and secondary valencies. All the chloride ions are non-ionisable and will not be precipitated by the addition of AgNO3. Therefore, the coordination compound behaves as neutral non- conducting molecule.
It may be formulated as [CoCl3(NH3)3] and does not ionise.
[CoCl3(NH3)3]⇔ Does not ionise
![structure of [CoCl3(NH3)3]](https://classnotes.org.in/wp-content/uploads/CoCl3NH33-300x282.jpeg)
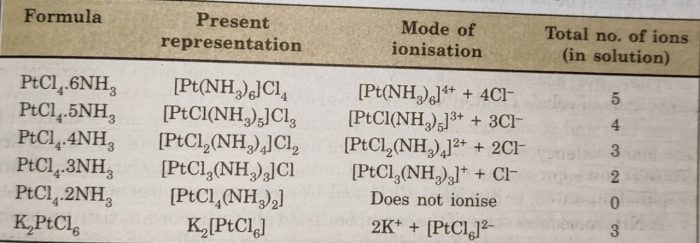
Behaviour of Coordination Compounds of Platinum
Leave a Reply