Contents
Terms used in Coordination compounds
Coordination Entity or Complex lon
A coordination entity constitutes a central metal atom or ion bonded to a fixed number of oppositely charged ions or neutral molecules. For example : [CoCl3(NH3)3] is a coordination entity, in which the cobalt (+3) ion is surrounded by three ammonia molecules and three negatively charged chloride ions. Other examples are [Ni(CO)4], [Ni(NH3)6]2+, [Fe(CN)6]4–, [PLCl4]2-, NiCl2(H2O)4] etc.
The central metal atom and the molecules or ions bonded to it i.e. coordination entity also represents coordination sphere. This part of the complex behaves as one unit and is non-ionizable. It is generally written in square brackets, [].
The ionizable groups (or ions) are written outside the brackets and are called counter ions. For example: In the coordination compound [Co(NH3)4Cl2]Cl, the Coordination entity is [Co(NH3)4Cl2] and Cl– ions are counter ions.
A coordination compound contains, neutral or charged coordination entity (or complex ion).
For example: [Ni(NH3)6]Cl2 contains the complex ion [Ni(NH3)6]2+, [Cu(NH3)4]SO4 contains the complex ion [Cu(NH3)4]2+, (Ag(NH3)2]Cl contains the complex ion [Ag(NH3)2]+ and so on.
The coordination entity (or complex ion) complex ion may be positively charged or negatively charged or a neutral species.
Cationic complex: A complex ion or coordination entity which has a net positive charge is called cationic complex.
Anionic complex: A complex ion or coordination entity which has a net negative charge is called anionic complex.
Neutral complex: A complex or coordination entity which has no net charge is called a neutral complex or simply a complex.
For example: [CoCl3(NH3)3] , [Ni(CO)4], [PtCl2(NH3)2)
Central atom or ion and Ligands
The neutral molecules or ions bound to the central atom or ion in the coordination entity are called ligands.
In the complex ion [Ni(NH3)6]2+ the Ni2+ ion is the central ion and the molecules of ammonia are the ligands. Similarly, in the complex ion [Co(NH3)5Cl]2+ , the Co3+ ion is the central ion while the ammonia molecules and chloride ions are the ligands.
|
[Ni(NH3)6]2+ Central ion : Ni2+
Ligands : NH3 molecules
[CoCl(NH3)5]2+ Central ion : Co3+
Ligands : NH3 molecules and Cl¯ ion
[Fe(CN)6]3– Central ion : Fe3+
Ligands : CN¯
[NiCl2(H2O)4] Central ion : Ni2+
Ligands : Cl¯ and H2O
|
The central metal ion should have vacant orbitals, the ligands should have lone pairs of electrons in their outermost orbitals which can be donated to the central ion.
The combination of a Lewis acid (the central metal atom or ion) with a number of Lewis bases (ligands). The atom in the ligand which can donate the electron pair is called donor atom or coordinating atom. For example: In ammonia, (←:NH3), nitrogen is the donor atom and in water, ( ←:OH2) oxygen is the donor atom.
Thus, ligand is an atom or molecule or ion which is capable of donating a pair of electrons to the central metal or ion and forms a coordinate bond with it.
Types of Ligands
(a) Unidentate or monodentate ligands: Ligands which can coordinate to the central ion through only one donor atom, are known as unidentate or monodentate ligands.
(b) Didentate or bidentate ligands: Ligands which have two donor atoms and therefore, can coordinate to the central ion at two positions, are called didentate or bidentate ligands.The examples of bidentate ligands are :
Oxalate ion is abbreviated as ox and ethane-1,2-diamine (ethylenediamine) is abbreviated as en.
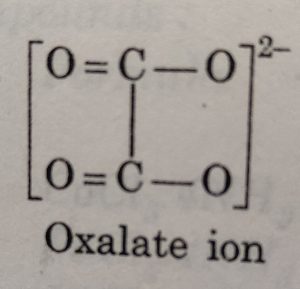
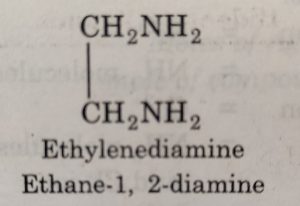
Glycinate ion, CH2(NH2)COO¯(abbreviated as gly) contains two different donor atoms N and O.
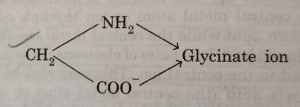
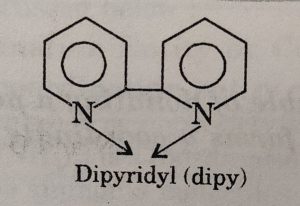
Similarly, 2, 2-dipyridyl (abbreviated as dipy) , orthophenanthroline or 1, 10-phenanthroline (abbreviated as phen) act as didentate ligands.
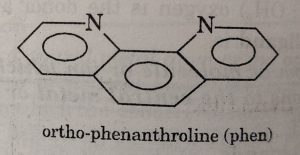
(1) The didentate ligands in which the two coordinating groups are same are called symmetrical didentate ligands. For example: ethylenediamine, oxalato ion etc.
(2) The didentate ligands in which the two coordinating groups are different are called unsymmetrical didentate ligands. For example: glycinate ion (NH2CH2COO¯).
(c) Polydentate ligands: Ligands having more than two donor atoms present in the molecule are called polydentate ligands. These may be called tri or terdentate (three), tetradentate (four), pentadentate (five) and hexadentate (six) ligands depending upon the number of donor atoms present in their molecules.For example:
(1) diethylene triamine (abbreviated as dien) acts as terdentate ligand having three donor N atoms.
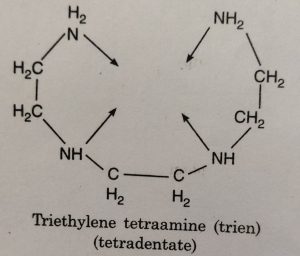
(2) triethylene tetraamine (abbreviated as trien) acts as tetradentate ligand having four donor N atoms and ethylenediamine triacetate ion acts as pentadentate ligand having two N atoms and three O donor atoms.
(3) Ethylenediaminetetraacetate ion (abbreviated as EDTA), is an important hexadentate ligand. It binds through two nitrogen and four oxygen atoms to a central metal ion.

Chelation and Denticity
When a di-or polydentate ligand uses its two or more donor atoms to bind the same central metal atom or ion, it is called chelation. The resulting complex has ring structure and the ligand coordinating through two or more donor groups is called chelating ligand.
Some common examples of chelating ligands are: carbonate ion, oxalate ion (ox2–), ethylenediamine (en), ortho-phenanthroline (ph), ethylenediaminetetra acetate ion (EDTA), etc. The complex is called chelate.
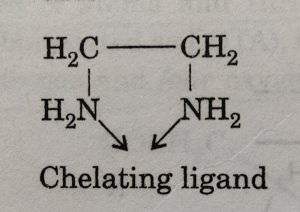
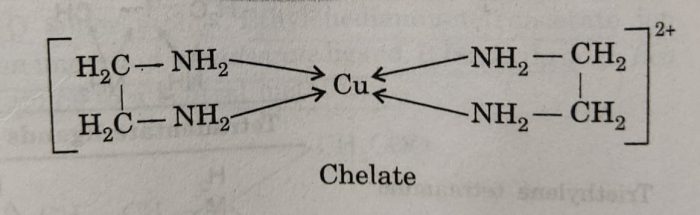
For example: when a bidentate ligand such as ethylenediamine attaches to Cu2+ ion through two amino groups and forms a ring structure, it is called a chelating ligand. The resulting complex ion [Cu(NH2CH2CH2NH2)2]2+ or [Cu(en)2]2+ is called a chelate.
The number of ligating groups indicate the denticity of the ligand. Some common examples are :-
(1) Didentate Chelation
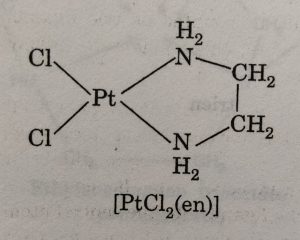
The chelating ligand coordinates through two sigma electron pair donor groups.For example: [PtCl2(en)] where en represents the didentate ligand NH2CH2CH2NH2 (ethane-1, 2-diamine or ethylenediamine).
(2) Terdentate Chelation
For example: in the coordination entity [PtCl(dien)]+, dien[N-(2-aminoethyl) ethane-1, 2-diamine] is a terdentate ligand.
![[PtCl(dien)]+](https://classnotes.org.in/wp-content/uploads/PtCldien-300x252.jpeg)
(3) Tetradentate Chelation
For example: in [Pt(trien)]2+, trien [N,N’-bis-(2-aminoethyl) ethane-1, 2-diamine] represents a tetradentate ligand.
![[Pt(trien)]2+](https://classnotes.org.in/wp-content/uploads/Pttrien2-300x233.jpeg)
Ambident ligands
The monodentate ligands which can coordinate with the central atom through more than one site are called ambidentate ligands. These ligands contain more than one coordinating atoms in their molecules.
M ← 0-N = 0 nitrito or nitrito-O
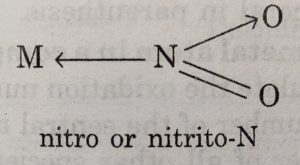
Coordination Number
The ligands in a coordination compound are attached to the central metal ion through coordination bonds. The total number of ligands attached to a central metal ion is called the coordination number of that ion. Coordination number is the number of ligands in the coordination sphere of the complex compound.
For example: The coordination number of central metal ion in different complexes are :
The molecules or ions present outside the square bracket (coordination sphere) are not counted in the coordination number because these are not bonded through coordinate bonds to the metal ion.
Charge of a Complex ion
The charge carried by a complex ion is the algebraic sum of charges carried by the central ion and the ligands coordinated to it.
For example:
Oxidation number or oxidation state of the central metal atom
The oxidation number of the central atom is the charge it would carry if all the ligands are removed along with the electron pairs that are shared with the central atom. For example: the oxidation number of cobalt in [Co(NH3)6]3+ is +3. Oxidation number is represented by a Roman numeral in parenthesis.
Calculation of oxidation number of central metal atom in a complex
(1) Knowing the charge of the complex ion, we can calculate the oxidation number of the central metal atom.
(2) The oxidation number of the central metal atom is assumed to be x and the oxidation number of all other species are substituted.
(3) The sum of the oxidation numbers is equated to the total charge of the complex and the value of x is calculated.
Examples :
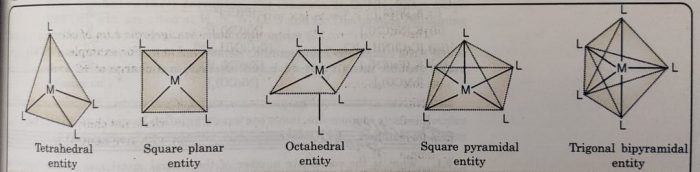
Coordination Polyhedron
Coordination polyhedron is the spatial arrangement of the ligand atoms which are directly attached to the central atom.
Homoleptic and Heteroleptic Complexes
Complexes in which the metal is bound to only one kind of donor groups are called homoleptic complexes.
For example: [Ni(NH3)6]3+, [Co(NH3)6]3+,[Fe(CN)6]4- are homoleptic complexes.
The complexes in which the metal is bound to more than one kind of donor groups are called heteroleptic complexes. Examples of heteroleptic complexes are [NiCl2(H2O)4], [CoCl2(NH3)4]+ etc.
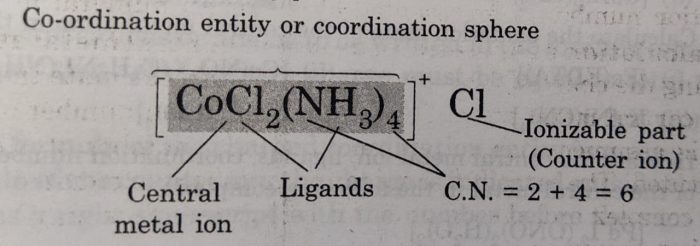
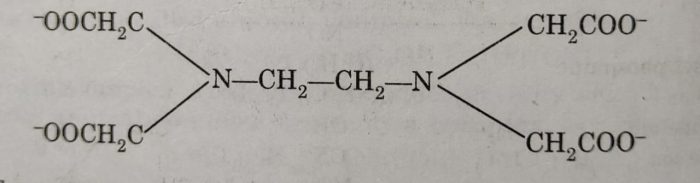
Leave a Reply