IUPAC Nomenclature of Coordination Compounds
Contents
- 1 Rules for Writing Formula
- 2 The ligands in coordination entity are arranged as
- 3 Rules for naming the coordination compounds
- 3.1 (1) Order of naming ions
- 3.2 (2) Naming the coordination entity
- 3.3 (3) Names of ligands
- 3.4 (4) Order of naming ligands
- 3.5 (5) Numerical prefixes to indicate number of ligands
- 3.6 (6) Ending of names
- 3.7 (7) Oxidation state of central metal ion
- 3.8 (8) Point of attachment
- 3.9 (9) Naming geometrical isomers
- 3.10 (10) Naming optical isomers
Rules for Writing Formula
The formula of a compound is a shorthand method used to provide basic information about the constitution of a compound in a concise and convenient manner.
(1) The formula of the cation whether simple or complex is written first followed by that of the anion.
(2) The coordination entity is written in square brackets.
(3) The sequence of symbols within the coordination entity is : first the symbol of the central metal atom followed by ligands in alphabetical order.
The ligands in coordination entity are arranged as
(a) The different ligands are arranged alphabetically according to the first symbol of their formulae. For example, H2O , NH3, NO3¯, SO42- and OH¯ etc. are cited at H, N, N, O, S and O.
(b) When the two ligands have same defining atom, the ligand with fewer such atoms is cited first followed by the ligand having more atoms. For example, NH3 precedes N2
(c) If the numbers of defining atoms are equal, subsequent symbol decides the sequence.
For example NH2¯ precedes NO2¯ because H comes before O.
(d) Polydentate ligands are also listed alphabetically. In case of abbreviated ligand, the first letter of the abbreviation is used to determine the position of the ligand in alphabetical order.
(e) The formula for the co-ordination entity, whether charged or not, is enclosed in square brackets. Polyatomic ligands are enclosed in parentheses (), but all ligands are written without any separation in between.
(f) There should be no space between the representations of ionic species within the formula.
(g) Sometimes abbreviations are used for formulae of the ligands. These abbreviations should be in lower case and enclosed in parenthesis.
For example, py is used for pyridine and en is used for ethane-1, 2-diamine or ethylene diamine.
(h) The number of cations or anions to be written in the formula is calculated on the basis that total positive charge must be equal to total negative charge.
(i) When the formula of the charged coordination entity is written without the formula of the counter ion, the charge is indicated outside the square brackets as a right superscript with the number before the sign (+ or -).
For example,
Some common examples are :
Rules for naming the coordination compounds
(1) Order of naming ions
(2) Naming the coordination entity
(3) Names of ligands
The names of anionic ligands (organic or inorganic) end in o-. In general, if the anionic ligand name ends in -ide, -ite or -ate, the final ‘e’ is replaced by ’o’ giving-ido,-ito and-ato respectively. For inorganic anionic ligands containing numerical prefixes such as triphosphate enclosing marks () are added. The names of positive ligands end in -ium. The neutral ligands are named as such.
For example :
(ii) positive ligands end in -ium
(iii) neutral ligands are named as such
NH2CH2CH2NH2 ethane-1,2-diamine or ethylenediamine
dipyridyl (dipy)
However, there are a few exceptions in naming neutral ligands. For example,
(4) Order of naming ligands
(5) Numerical prefixes to indicate number of ligands
When the name of the ligand, includes the numerical prefix (di, tri, tetra), the prefixes bis, tris, tetrakis are used for two, three, four ligands, respectively. Such ligands are called complexligands.
For example: to indicate two simple ligands such as chloro, bromo, ammine, oxalato, etc., we use the prefix di but to indicate two complex ligands such as ethylenediamine we use the prefix bis (ethylenediamine) or bis (1,2-ethanediamine).
The name of the complex ligand is given in brackets.
For example :
(6) Ending of names
For example, the cationic complex [Co(NH3)6]Cl3 is named without characteristic ending of the name of the metal as :
[Co(NH3)6]Cl3 hexaamminecobalt (III) chloride
The coordination compound, K[PtCl5(NH3)] which contains the anionic complex [PtCl5(NH3)]¯ is named with ending of the name of the metal as -ate.
K[PtCl5(NH3)] potassium amminepentachloridoplatinate (IV)
Similarly, the anionic complex Ca2[Fe(CN)6] is named as calcium hexacyanoferrate (II).
[Co(SCN)4]2– tetrathiocyanatocobaltate(II) ion.
For anionic complexes the Latin names of certain metals are commonly used.
For example: ferrate for Fe, cuperate for Cu, argentate for Ag, aurate for Au, stannate for
However, if the complex is cationic or neutral the name of the metal is given as such e.g., iron for Fe, silver for Ag, gold for Au, copper for Cu, etc.
For example:
(7) Oxidation state of central metal ion
(i) CoCl(NH3)5]Cl2
(ii) K3[Fe(CN)6]
(iii) [Co(H2NCH2CH2NH2)3] (SO4)3
(iv) [Ag(NH3)2] [Ag(CN)2]
Both cation and anion are complexes. The oxidation state of silver in both cationic and anionic complexes is +1. The name of the complex is diamminesilver (I) dicyanoargentate (I)
(8) Point of attachment
NO2¯ (through N)
SCN¯ (through S) thiocyanato
For example,
[Co(NO2)3(NH3)3] triamminetrinitrito-N-cobalt(
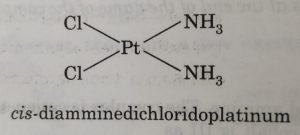
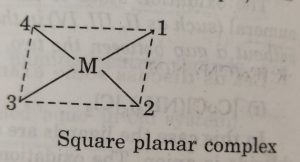
(9) Naming geometrical isomers
For example: in square planar complexes shown below
In naming these complexes, cis or trans is written before the names of these compounds. Similarly, for octahedral complexes, cis- and trans are used in isomerism in coordination compounds.
(10) Naming optical isomers
Optically active compounds are designated by the symbols (+) or d -for dextrorotatory and (-) or l- for laevorotatory.
For example : d- K3[Cr(C2O4)3] Potassium (+) trioxalatochromate (III)
Leave a Reply