The rate of a reaction can be increased by raising the temperature. However, temperature can be raised within certain limits because in certain cases, the reactants become unstable at higher temperatures and decompose.
Many reactions are made to proceed at an increased rate by the presence of some other substance.
For example : a mixture of H2 and O2 does not react at room temperature. However, in the presence of finely divided platinum, the reaction becomes quite vigorous.
Manganese dioxide, a black powder speeds up the thermal decomposition of potassium chlorate.
2 KClO3 (s) ——–> 2KCl (s) + 3 O2
Catalysts
The substances which accelerate the rate of reactions without itself undergoing permanent change are called catalysts.
The substances which increase the rate of a reaction and can be recovered chemically unchanged in mass and composition after the reaction are called catalysts.
The phenomenon of increasing the rate of a reaction by the use of catalyst is called catalysis. A catalyst is not consumed in the reaction.
In a catalysed reaction the catalyst is used in one step and is regenerated in subsequent step and thus, it is used up again and again without undergoing any permanent change.
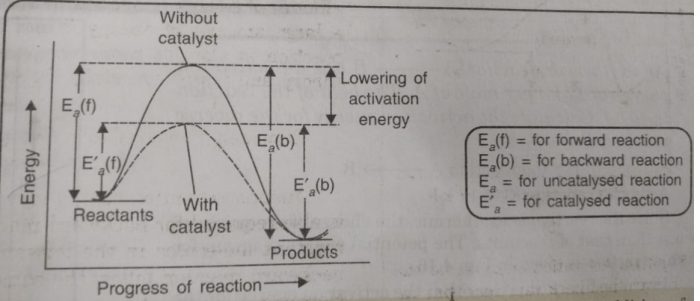
Catalyst provides an entirely new path for the reaction in which the reactants are converted to products quickly. The catalyst forms a new activated complex of lower potential energy. This means that the activation energy becomes lower for the catalysed reaction than that for uncatalysed reaction. The fraction of the total number of collisions possessing lower activation energy increased and hence, the rate of reaction also increase.
The solid line shows the path for uncatalysed reaction and dotted line shows the path adopted by catalysed reaction. Consider a hypothetical reaction
A + B ——–> AB
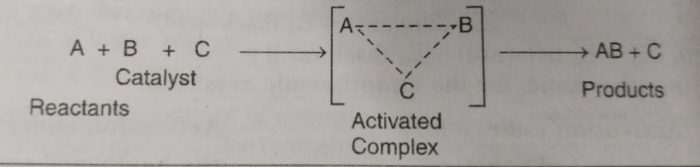
The reaction proceeds through the formation of activated complex
A+B ——> [A…B] ——> AB
Addition of a catalyst C result into the formation of new activated complex of lower activation energy.
The Function of Catalyst
(1) A catalyst may undergo intermediate physical changes and it may even form temporary chemical bonds with the reactants but it is recovered unchanged in original form at the end of the reaction.
(2) A catalyst speeds up the reaction but it does not shift the position of equilibrium. The presence of a catalyst reduces the height of barrier by providing an alternative path for the reaction and lowers the activation energy. The lowering in activation energy is to the same extent for the forward as well as for the backward reaction.
(3) Catalysts are highly specific in nature. A catalyst which can catalyse one reaction may have no effect on another reaction even, if that reaction is very similar.
(4) The catalyst does not change ΔE (or ΔH) of the reaction.
very helpful Maam