Discovery of Electron
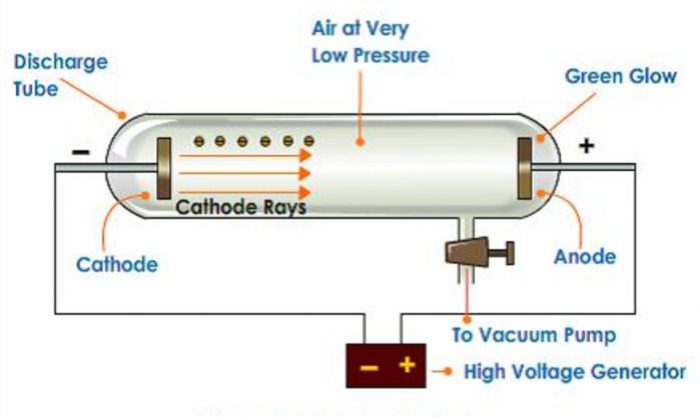
1) It consist of a long cylindrical tube sealed at both ends and is fitted with two metal electrodes.
2) The electrodes are connected to source of high voltage.
3) The tube is connected to a vacuum pump as to increase or decrease the pressure.
4) The discharge tube is filled with air.
Experiment
He passed electricity at high voltage through a gas at very low pressure taken in discharge tube.
Characteristics of Electrons
(1)The charge on an electron is 1.6×10−19 C.This charge is found to be smallest negative charge carried by any particle.So it is taken as unit negative charge.
(2)The absolute mass of electron is 9.1 x 10-31 kg .
Nice information.can I get pdf ??
Dear Ma’am,
I’m very much thankful to you, thanks for helping me to complete my notes… You are a great teacher Ma’am.. and again thanks Ma’am
Your Sincerely
– Bhumika Mahala
Very nice . I loved it, thank you for uploading it.
Thank you so much to you for making it easy to make notes of it
Dear sir/madam I am very thankful to you for your information
I appreciate the efforts…its helpful