|
Latent heat | Matter in our Surroundings | Class 9 |
Question 1 What is latent heat ?
Question 2 Define the term Latent heat of fusion of a solid ?
Question 3 Define the term latent heat of Vaporisation of a liquid?
question 4 Why does the temperature remain constant during the melting of ice even though heat is supplied continuously ?
Question 5 Why does the temperature remain constant during the boiling of water even though heat is supplied continuously ?
Question 6 What is the value of latent heat of vaporisation of water ?
Question 7 What is the value of latent heat of fusion of ice ?
Latent Heat
The heat energy which has to be supplied to change the state of a substance is called latent heat.
Latent heat does not raise the temperature. But the latent heat has always to be supplied to change the state of a substance. It is called latent heat because it becomes hidden in the substance undergoing the change of state and does not show its presence by raising the temperature. The latent heat which we supply is used up in overcoming the forces of attraction between the particles of a substance during the change of state.
The latent heat does not increase the kinetic energy of the particles of the substance. And since there is no increase in the kinetic energy of the particles, the temperature of a substance does not rise during the change of state.
Latent heat is of two types:
1) Latent Heat of Fusion
2) Latent Heat of Vaporisation
Latent Heat of Fusion
The latent heat of fusion or melting of solid is the quantity of heat in joules required to convert 1 kg of solid to liquid, without any change in temperature.
The latent heat of fusion of ice is 3.34 ⨰ 105 /kg.
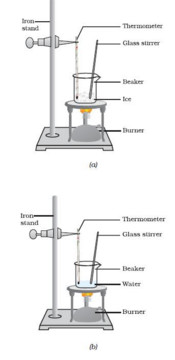
We take some crushed ice in a beaker and suspend a thermometer in it. We note the temperature of ice. It is found to be 0. We now heat the ice gently by using a small flame of a burner. On heating, ice starts melting to form water. We keep on recording the temperature of melting ice on the thermometer every minute. As more heat is given, more ice melts to form water but the thermometer reading remains 0.As long as there remains even a little of ice in the beaker, the thermometer does not rise, it remains constant at 0.This shows that there is no rise in temperature during the melting of ice. It is only when all the ice has melted that the temperature of water starts rising on further heating. The heat which is going into ice but not increasing its temperature, is the energy required to change the state of ice from solid to liquid.
Ice has strong inter particle forces of attraction. The heat which we supply to ice during melting is used up to overcome the forces of attraction so that they become somewhat loose. This heat does increases kinetic energy of particles and hence no rise in temperature take place. But when all the ice has melted to form water, further heating increases the kinetic energy of particles due to which the temperature increases.
Latent heat of Vaporisation
It is the quantity of heat in joules required to convert 1 kg of liquid to vapour or gas, without any change in temperature.
For Example: latent heat of vaporisation of water is 22.5 ⨰105 J/Kg
Take some water in a beaker and suspend a thermometer in it. We heat this water by using a small burner and note its temperature after every minute. As heat is given, the temperature of water rises gradually until 100 is reached. At the temperature of 100 water boils and start changing into steam. As more heat is given to water, more steam is formed but the thermometer reading remains at 100 showing that there is no rise in temperature during the boiling water. Thus, once the water begun to boil, the temperature remains constant at 100 until all the water has changed into steam. The heat which is going into boiling water but not increasing its temperature is the energy required to change the state of water from liquid to gas.
Particles of water attract each other. The heat energy which we supply during boiling is used to overcome forces of attraction between water particles so that they become totally free and change into a gas. This latent heat does not increase the kinetic energy and hence no rise in temperature take place.
this notes should be helpful for examination.
yes very helpful for upcoming examinations
This is fantastic Madam. Very helpful in understanding..
VERY VERY HELPHUL FOR MY PHYSICS PROJECT, THANKS A LOT.
Amazing work! So crisp and easily understandable:)
Thank you!
Very useful for my upcoming exam!
The work is very helpful and in very easy or simple language thanks for help us